Intraflagellar Transport Proteins as Regulators of Primary Cilia Length
- PMID: 34095126
- PMCID: PMC8170031
- DOI: 10.3389/fcell.2021.661350
Intraflagellar Transport Proteins as Regulators of Primary Cilia Length
Abstract
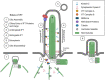
Primary cilia are small, antenna-like organelles that detect and transduce chemical and mechanical cues in the extracellular environment, regulating cell behavior and, in turn, tissue development and homeostasis. Primary cilia are assembled via intraflagellar transport (IFT), which traffics protein cargo bidirectionally along a microtubular axoneme. Ranging from 1 to 10 μm long, these organelles typically reach a characteristic length dependent on cell type, likely for optimum fulfillment of their specific roles. The importance of an optimal cilia length is underscored by the findings that perturbation of cilia length can be observed in a number of cilia-related diseases. Thus, elucidating mechanisms of cilia length regulation is important for understanding the pathobiology of ciliary diseases. Since cilia assembly/disassembly regulate cilia length, we review the roles of IFT in processes that affect cilia assembly/disassembly, including ciliary transport of structural and membrane proteins, ectocytosis, and tubulin posttranslational modification. Additionally, since the environment of a cell influences cilia length, we also review the various stimuli encountered by renal epithelia in healthy and diseased states that alter cilia length and IFT.
Keywords: IFT-A; IFT-B; cilia disassembly; ciliogenesis; ectocytosis; kidney; posttranslational modification.
Copyright © 2021 Wang, Jack, Wang, Kavanaugh, Maser and Tran.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling.Curr Top Dev Biol. 2008;85:23-61. doi: 10.1016/S0070-2153(08)00802-8. Curr Top Dev Biol. 2008. PMID: 19147001 Review.
-
A differential cargo-loading model of ciliary length regulation by IFT.Curr Biol. 2013 Dec 16;23(24):2463-71. doi: 10.1016/j.cub.2013.10.044. Epub 2013 Dec 5. Curr Biol. 2013. PMID: 24316207 Free PMC article.
-
Genetic interaction of mammalian IFT-A paralogs regulates cilia disassembly, ciliary entry of membrane protein, Hedgehog signaling, and embryogenesis.FASEB J. 2020 May;34(5):6369-6381. doi: 10.1096/fj.201902611R. Epub 2020 Mar 13. FASEB J. 2020. PMID: 32167205 Free PMC article.
-
Protein transport in growing and steady-state cilia.Traffic. 2017 May;18(5):277-286. doi: 10.1111/tra.12474. Epub 2017 Mar 29. Traffic. 2017. PMID: 28248449 Free PMC article. Review.
-
Tubulin transport by IFT is upregulated during ciliary growth by a cilium-autonomous mechanism.J Cell Biol. 2015 Jan 19;208(2):223-37. doi: 10.1083/jcb.201409036. Epub 2015 Jan 12. J Cell Biol. 2015. PMID: 25583998 Free PMC article.
Cited by
-
Loss of Ift74 Leads to Slow Photoreceptor Degeneration and Ciliogenesis Defects in Zebrafish.Int J Mol Sci. 2021 Aug 28;22(17):9329. doi: 10.3390/ijms22179329. Int J Mol Sci. 2021. PMID: 34502236 Free PMC article.
-
Role of U11/U12 minor spliceosome gene ZCRB1 in Ciliogenesis and WNT Signaling.bioRxiv [Preprint]. 2024 Aug 10:2024.08.09.607392. doi: 10.1101/2024.08.09.607392. bioRxiv. 2024. PMID: 39149385 Free PMC article. Preprint.
-
The Role of Sonic Hedgehog in Human Holoprosencephaly and Short-Rib Polydactyly Syndromes.Int J Mol Sci. 2021 Sep 12;22(18):9854. doi: 10.3390/ijms22189854. Int J Mol Sci. 2021. PMID: 34576017 Free PMC article. Review.
-
The ciliary gene INPP5E confers dorsal telencephalic identity to human cortical organoids by negatively regulating Sonic hedgehog signaling.Cell Rep. 2022 May 17;39(7):110811. doi: 10.1016/j.celrep.2022.110811. Cell Rep. 2022. PMID: 35584663 Free PMC article.
-
Evolution of glutamatergic signaling and synapses.Neuropharmacology. 2021 Nov 1;199:108740. doi: 10.1016/j.neuropharm.2021.108740. Epub 2021 Jul 31. Neuropharmacology. 2021. PMID: 34343611 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

