Beyond Conventional: The New Horizon of Targeted Therapy for the Treatment of Advanced Non Small Cell Lung Cancer
- PMID: 34094913
- PMCID: PMC8176852
- DOI: 10.3389/fonc.2021.632256
Beyond Conventional: The New Horizon of Targeted Therapy for the Treatment of Advanced Non Small Cell Lung Cancer
Abstract
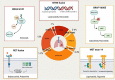
In the last few years the advent of targeted therapies against oncogenic drivers significantly improved the survival of non small cell lung cancer (NSCLC) patients with a favourable toxicity profile. Therefore, genetic testing, including at least EGFR mutations and ALK/ROS1 rearrangements, should be performed in all NSCLC patients (in particular with adenocarcinoma) who received a diagnosis of advanced disease. This review focuses on novel druggable oncogenic drivers, such as MET exon 14 mutations/MET amplification, RET fusions, BRAF V600E mutations, KRAS G12C mutations, NTRK rearrangements, and HER2 alterations.
Keywords: MET; genetic testing; non small cell lung cancer; oncogenic drivers; targeted therapies.
Copyright © 2021 Tartarone, Lapadula, Di Micco, Rossi, Ottanelli, Marini, Giorgione, Ferrari, Catalano, Voltolini, Mini and Roviello.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Efficacy of immunotherapy in patients with oncogene-driven non-small-cell lung cancer: a systematic review and meta-analysis.Ther Adv Med Oncol. 2024 Feb 27;16:17588359231225036. doi: 10.1177/17588359231225036. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 38420602 Free PMC article. Review.
-
Targeted Therapy in Advanced and Metastatic Non-Small Cell Lung Cancer. An Update on Treatment of the Most Important Actionable Oncogenic Driver Alterations.Cancers (Basel). 2021 Feb 15;13(4):804. doi: 10.3390/cancers13040804. Cancers (Basel). 2021. PMID: 33671873 Free PMC article. Review.
-
New horizons for uncommon mutations in non-small cell lung cancer: BRAF, KRAS, RET, MET, NTRK, HER2.World J Clin Oncol. 2022 Apr 24;13(4):276-286. doi: 10.5306/wjco.v13.i4.276. World J Clin Oncol. 2022. PMID: 35582653 Free PMC article. Review.
-
Beyond ALK and ROS1: RET, NTRK, EGFR and BRAF gene rearrangements in non-small cell lung cancer.Transl Lung Cancer Res. 2017 Oct;6(5):550-559. doi: 10.21037/tlcr.2017.08.02. Transl Lung Cancer Res. 2017. PMID: 29114471 Free PMC article. Review.
-
Beyond EGFR and ALK inhibition: unravelling and exploiting novel genetic alterations in advanced non small-cell lung cancer.Cancer Treat Rev. 2015 May;41(5):401-11. doi: 10.1016/j.ctrv.2015.03.009. Epub 2015 Mar 28. Cancer Treat Rev. 2015. PMID: 25842168 Review.
Cited by
-
Short report: Performance evaluation of the Idylla™ KRAS and EGFR mutation tests on paraffin-embedded cytological NSCLC samples.Diagn Pathol. 2021 Aug 3;16(1):70. doi: 10.1186/s13000-021-01121-3. Diagn Pathol. 2021. PMID: 34344387 Free PMC article.
-
Assessing the prognostic value of KRAS mutation combined with tumor size in stage I-II non-small cell lung cancer: a retrospective analysis.Front Oncol. 2024 May 31;14:1396285. doi: 10.3389/fonc.2024.1396285. eCollection 2024. Front Oncol. 2024. PMID: 38884086 Free PMC article.
-
Ubiquitylation of RUNX3 by RNA-binding ubiquitin ligase MEX3C promotes tumorigenesis in lung adenocarcinoma.J Transl Med. 2024 Feb 29;22(1):216. doi: 10.1186/s12967-023-04700-8. J Transl Med. 2024. PMID: 38424632 Free PMC article.
-
NTRK-fusions in pediatric thyroid tumors: Current state and future perspectives.Cancer Genet. 2022 Jun;264-265:23-28. doi: 10.1016/j.cancergen.2022.02.009. Epub 2022 Mar 6. Cancer Genet. 2022. PMID: 35290879 Free PMC article. Review.
References
-
- Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim DW, et al. . Updated Overall Survival and Final Progression-Free Survival Data for Patients With Treatment-Naive Advanced ALK-positive non-Small-Cell Lung Cancer in the ALEX Study. Ann Oncol (2020) 31:1056–64. 10.1016/j.annonc.2020.04.478 - DOI - PubMed
-
- Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH, et al. . Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med (2018) 142:321–46. 10.5858/arpa.2017-0388-CP - DOI - PubMed
-
- Kalemkerian GP, Narula N, Kennedy EB, Biermann WA, Donington J, Leighl NB, et al. . Molecular Testing Guideline for the Selection of Patients With Lung Cancer for Treatment With Targeted Tyrosine Kinase Inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J Clin Oncol (2018) 36:911–9. 10.1200/JCO.2017.76.7293 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous