Voltage-gated potassium channels are involved in oxymatrine-regulated islet function in rat islet β cells and INS-1 cells
- PMID: 34094027
- PMCID: PMC8143713
- DOI: 10.22038/ijbms.2021.52449.11850
Voltage-gated potassium channels are involved in oxymatrine-regulated islet function in rat islet β cells and INS-1 cells
Abstract
Objectives: Oxymatrine can regulate glucose metabolism. But the underlying mechanisms remain unclear. We investigated the relationship of oxymatrine and voltage-gated potassium (Kv) channel in rat islet β cells and INS-1 cells.
Materials and methods: Insulin secretion and Kv channel currents were tested by radioimmunoassay and patch-clamp technique, respectively. The INS-1 cell viability was detected using cell counting kit-8 experiments. Flowcytometry analysis and western blot were employed for cell apoptosis and protein levels, respectively. INS-1 cell proliferation was assessed by the 5-Ethynyl-2'- deoxyuridine method.
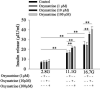
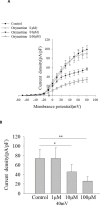
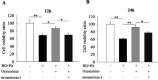
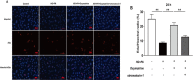
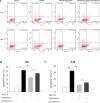
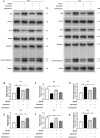
Results: Oxymatrine potentiated insulin secretion at high glucose (P<0.01 vs 11.1 G, P<0.01 vs 16.7 G) and inhibited KV currents at 40 mV (45.73±15.34 pA/pF for oxymatrine, 73.80±19.23 pA/pF for control, P<0.05). After the INS-1 cells were treated with oxymatrine for 12 and 24 hr, KV2.1 channel protein was up-regulated (P<0.01 vs Control). At the same time, compared with the high glucose and high fat group, cell viability and proliferation ability were increased (P<0.01). The cell apoptotic rate was reduced, reaching 17.30%±1.00% at 12 hr and 10.35%±1.52% at 24 hr (P<0.01). These protective effects of oxymatrine were reversed by using Stromatoxin-1, a kv channel inhibitor.
Conclusion: The results indicate that oxymatrine can stimulate insulin secretion and decrease kv channel currents in islet β cells. Besides, oxymatrine also increases cell viability, proliferation, and reduces cell apoptosis in INS-1 cells. The effects of oxymatrine are related to kv channels. This finding provides new insight into the mechanisms of oxymatrine-regulated islet function.
Keywords: Apoptosis; Diabetes mellitus; Insulin secretion; Oxymatrine; Potassium channel; Voltage-gated.
Figures

Similar articles
-
Inhibition of voltage-gated potassium channels mediates uncarboxylated osteocalcin-regulated insulin secretion in rat pancreatic β cells.Eur J Pharmacol. 2016 Apr 15;777:41-8. doi: 10.1016/j.ejphar.2016.02.060. Epub 2016 Feb 27. Eur J Pharmacol. 2016. PMID: 26927753
-
The PLC/PKC/Ras/MEK/Kv channel pathway is involved in uncarboxylated osteocalcin-regulated insulin secretion in rats.Peptides. 2016 Dec;86:72-79. doi: 10.1016/j.peptides.2016.10.004. Epub 2016 Oct 14. Peptides. 2016. PMID: 27746193
-
Calcium-activated and voltage-gated potassium channels of the pancreatic islet impart distinct and complementary roles during secretagogue induced electrical responses.J Physiol. 2010 Sep 15;588(Pt 18):3525-37. doi: 10.1113/jphysiol.2010.190207. Epub 2010 Jul 19. J Physiol. 2010. PMID: 20643768 Free PMC article.
-
Voltage-dependent K(+) channels in pancreatic beta cells: role, regulation and potential as therapeutic targets.Diabetologia. 2003 Aug;46(8):1046-62. doi: 10.1007/s00125-003-1159-8. Epub 2003 Jun 27. Diabetologia. 2003. PMID: 12830383 Review.
-
Beta-cell ion channels: keys to endodermal excitability.Horm Metab Res. 1999 Aug;31(8):455-61. doi: 10.1055/s-2007-978774. Horm Metab Res. 1999. PMID: 10494870 Review.
Cited by
-
Oxymatrine inhibits the pyroptosis in rat insulinoma cells by affecting nuclear factor kappa B and nuclear factor (erythroid-derived 2)-like 2 protein/heme oxygenase-1 pathways.Korean J Physiol Pharmacol. 2022 May 1;26(3):165-174. doi: 10.4196/kjpp.2022.26.3.165. Korean J Physiol Pharmacol. 2022. PMID: 35477544 Free PMC article.
References
-
- International Diabetes Federation. IDF Diabetes Atlas-9th Edition. https://diabetesatlas.org/en / - PubMed
-
- Wang Z. Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch. 2004;448:274–286. - PubMed
-
- González C, Baez-Nieto D, Valencia I, Oyarzún I, Rojas P, Naranjo D, et al. K(+) channels: function-structural overview. Compr Physiol. 2012;2:2087–2149. - PubMed
-
- Leung YM. Voltage-gated K+ channel modulators as neuroprotective agents. Life Sci. 2010;86:775–780. - PubMed
LinkOut - more resources
Full Text Sources
