Effect of Voacamine upon inhibition of hypoxia induced fatty acid synthesis in a rat model of methyln-nitrosourea induced mammary gland carcinoma
- PMID: 34090331
- PMCID: PMC8180083
- DOI: 10.1186/s12860-021-00371-9
Effect of Voacamine upon inhibition of hypoxia induced fatty acid synthesis in a rat model of methyln-nitrosourea induced mammary gland carcinoma
Abstract
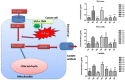
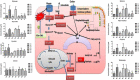
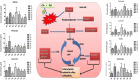
Background: In the present study, fatty acid synthesis is targeted to combat mammary gland carcinoma by activating prolyl hydroxylase-2 with Voacamine alone and in combination with Tamoxifen. It was hypothesized that the activation of prolyl hydroxylase-2 would inhibit the hypoxia-induced fatty acid synthesis and mammary gland carcinoma. Mammary gland carcinoma was induced with a single dose administration of N-methyl-N-nitrosourea (50 mg/kg,i.p.) and treatment with Voacamine and Tamoxifen 15 days after carcinogen administration.
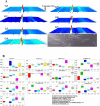
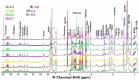
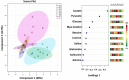
Results: At the end of the study, hemodynamic profiling of animals was recorded to assess the cardiotoxic potential of the drug. Blood serum was separated and subjected to nuclear magnetic resonance spectroscopy. Carmine staining and histopathology of mammary gland tissue were performed to evaluate the anti-angiogenic potential of the drug. The antioxidant potential of the drug was measured with antioxidant markers. Western blotting was performed to study the effect of the drug at the molecular level.
Conclusion: Results of the study have shown that Voacamine treatment stopped further decrease in body weight of experimental animals. The hemodynamic study evidenced that Voacamine at a low dose is safe in cardiac patients. Microscopic evaluation of mammary gland tissue documented the anti-angiogenic potential of Voacamine and Tamoxifen therapy. Perturbed serum metabolites were also restored to normal along with antioxidant markers. Immunoblotting of mammary gland tissue also depicted restoration of proteins of the hypoxic and fatty acid pathway. Conclusively, Voacamine and its combination with Tamoxifen activated prolyl hydroxylase-2 to combat mammary gland carcinoma.
Keywords: Fatty acid synthase; Hypoxia-inducible factor-1α; Mammary gland carcinoma; Prolyl hydroxylase-2; Tamoxifen.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


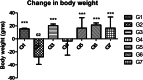


Similar articles
-
Repurposing Combination Therapy of Voacamine With Vincristine for Downregulation of Hypoxia-Inducible Factor-1α/Fatty Acid Synthase Co-axis and Prolyl Hydroxylase-2 Activation in ER+ Mammary Neoplasia.Front Cell Dev Biol. 2021 Nov 18;9:736910. doi: 10.3389/fcell.2021.736910. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34869321 Free PMC article.
-
Activation of prolyl hydroxylase-2 for stabilization of mitochondrial stress along with simultaneous downregulation of HIF-1α/FASN in ER + breast cancer subtype.Cell Biochem Funct. 2019 Jun;37(4):216-227. doi: 10.1002/cbf.3389. Epub 2019 Apr 5. Cell Biochem Funct. 2019. PMID: 30950543
-
PHD-2 activation: a novel strategy to control HIF-1α and mitochondrial stress to modulate mammary gland pathophysiology in ER+ subtype.Naunyn Schmiedebergs Arch Pharmacol. 2019 Oct;392(10):1239-1256. doi: 10.1007/s00210-019-01658-7. Epub 2019 Jun 1. Naunyn Schmiedebergs Arch Pharmacol. 2019. PMID: 31154466
-
Prolyl hydroxylase mediated inhibition of fatty acid synthase to combat tumor growth in mammary gland carcinoma.Breast Cancer. 2016 Nov;23(6):820-829. doi: 10.1007/s12282-016-0683-6. Epub 2016 Mar 7. Breast Cancer. 2016. PMID: 26951539 Review.
-
Atlas and histologic classification of tumors of the rat mammary gland.J Mammary Gland Biol Neoplasia. 2000 Apr;5(2):187-200. doi: 10.1023/a:1026443305758. J Mammary Gland Biol Neoplasia. 2000. PMID: 11149572 Review.
Cited by
-
Repurposing Combination Therapy of Voacamine With Vincristine for Downregulation of Hypoxia-Inducible Factor-1α/Fatty Acid Synthase Co-axis and Prolyl Hydroxylase-2 Activation in ER+ Mammary Neoplasia.Front Cell Dev Biol. 2021 Nov 18;9:736910. doi: 10.3389/fcell.2021.736910. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34869321 Free PMC article.
-
Regulation of Transactivation at C-TAD Domain of HIF-1α by Factor-Inhibiting HIF-1α (FIH-1): A Potential Target for Therapeutic Intervention in Cancer.Oxid Med Cell Longev. 2022 May 10;2022:2407223. doi: 10.1155/2022/2407223. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35592530 Free PMC article. Review.
-
NF-κB mediated regulation of tumor cell proliferation in hypoxic microenvironment.Front Pharmacol. 2023 Feb 20;14:1108915. doi: 10.3389/fphar.2023.1108915. eCollection 2023. Front Pharmacol. 2023. PMID: 36891273 Free PMC article. Review.
-
Hypoxia induced lactate acidosis modulates tumor microenvironment and lipid reprogramming to sustain the cancer cell survival.Front Oncol. 2023 Jan 25;13:1034205. doi: 10.3389/fonc.2023.1034205. eCollection 2023. Front Oncol. 2023. PMID: 36761981 Free PMC article. Review.
-
Roxadustat (FG-4592) Facilitates Recovery From Renal Damage by Ameliorating Mitochondrial Dysfunction Induced by Folic Acid.Front Pharmacol. 2022 Feb 25;12:788977. doi: 10.3389/fphar.2021.788977. eCollection 2021. Front Pharmacol. 2022. PMID: 35280255 Free PMC article.
References
-
- Aranda-Gutierrez A, Diaz-Perez HM. Histology, Mammary Glands. 2019. - PubMed
-
- Dey S, Roy S, Deb N, Sen KK, Besra SE. Anti-carcinogenic activity of Ruellia tuberosa L.(Acanthaceae) leaf extract on hepatoma cell line & increased superoxide dismutase activity on macrophage cell lysate. Int J Pharm Pharm Sci. 2013;5(3):854–861.
-
- Mishra A, Roy S, Maity S, Yadav RK, Keshari A, Saha S. Antiproliferative effect of flower extracts of Spilanthes paniculata on hepatic carcinoma cells. Int J Pharm Sci. 2015;7:130–134.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

