Adipocyte-Specific Deletion of Lamin A/C Largely Models Human Familial Partial Lipodystrophy Type 2
- PMID: 34088712
- PMCID: PMC8576431
- DOI: 10.2337/db20-1001
Adipocyte-Specific Deletion of Lamin A/C Largely Models Human Familial Partial Lipodystrophy Type 2
Abstract
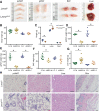
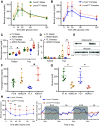
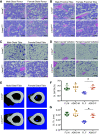
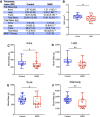
Mechanisms by which autosomal recessive mutations in Lmna cause familial partial lipodystrophy type 2 (FPLD2) are poorly understood. To investigate the function of lamin A/C in adipose tissue, we created mice with an adipocyte-specific loss of Lmna (Lmna ADKO). Although Lmna ADKO mice develop and maintain adipose tissues in early postnatal life, they show a striking and progressive loss of white and brown adipose tissues as they approach sexual maturity. Lmna ADKO mice exhibit surprisingly mild metabolic dysfunction on a chow diet, but on a high-fat diet they share many characteristics of FPLD2 including hyperglycemia, hepatic steatosis, hyperinsulinemia, and almost undetectable circulating adiponectin and leptin. Whereas Lmna ADKO mice have reduced regulated and constitutive bone marrow adipose tissue with a concomitant increase in cortical bone, FPLD2 patients have reduced bone mass and bone mineral density compared with controls. In cell culture models of Lmna deficiency, mesenchymal precursors undergo adipogenesis without impairment, whereas fully differentiated adipocytes have increased lipolytic responses to adrenergic stimuli. Lmna ADKO mice faithfully reproduce many characteristics of FPLD2 and thus provide a unique animal model to investigate mechanisms underlying Lmna-dependent loss of adipose tissues.
© 2021 by the American Diabetes Association.
Figures
Similar articles
-
The role of LMNA in adipose: a novel mouse model of lipodystrophy based on the Dunnigan-type familial partial lipodystrophy mutation.J Lipid Res. 2009 Jun;50(6):1068-79. doi: 10.1194/jlr.M800491-JLR200. Epub 2009 Feb 5. J Lipid Res. 2009. PMID: 19201734 Free PMC article.
-
FPLD2 LMNA mutation R482W dysregulates iPSC-derived adipocyte function and lipid metabolism.Biochem Biophys Res Commun. 2018 Jan 1;495(1):254-260. doi: 10.1016/j.bbrc.2017.11.008. Epub 2017 Nov 3. Biochem Biophys Res Commun. 2018. PMID: 29108996
-
Altered adipocyte differentiation and unbalanced autophagy in type 2 Familial Partial Lipodystrophy: an in vitro and in vivo study of adipose tissue browning.Exp Mol Med. 2019 Aug 2;51(8):1-17. doi: 10.1038/s12276-019-0289-0. Exp Mol Med. 2019. PMID: 31375660 Free PMC article.
-
Polycystic ovary syndrome in familial partial lipodystrophy type 2 (FPLD2): basic and clinical aspects.Nucleus. 2018;9(1):392-397. doi: 10.1080/19491034.2018.1509659. Nucleus. 2018. PMID: 30131000 Free PMC article. Review.
-
Molecular and Mechanobiological Pathways Related to the Physiopathology of FPLD2.Cells. 2020 Aug 23;9(9):1947. doi: 10.3390/cells9091947. Cells. 2020. PMID: 32842478 Free PMC article. Review.
Cited by
-
Deciphering the Clinical Presentations in LMNA-related Lipodystrophy: Report of 115 Cases and a Systematic Review.J Clin Endocrinol Metab. 2024 Feb 20;109(3):e1204-e1224. doi: 10.1210/clinem/dgad606. J Clin Endocrinol Metab. 2024. PMID: 37843397 Free PMC article.
-
Lipolysis of bone marrow adipocytes is required to fuel bone and the marrow niche during energy deficits.Elife. 2022 Jun 22;11:e78496. doi: 10.7554/eLife.78496. Elife. 2022. PMID: 35731039 Free PMC article.
-
Not Enough Fat: Mouse Models of Inherited Lipodystrophy.Front Endocrinol (Lausanne). 2022 Feb 18;13:785819. doi: 10.3389/fendo.2022.785819. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35250856 Free PMC article. Review.
-
Emerging roles of circular RNAs in osteoporosis.J Cell Mol Med. 2021 Oct;25(19):9089-9101. doi: 10.1111/jcmm.16906. Epub 2021 Sep 6. J Cell Mol Med. 2021. PMID: 34490735 Free PMC article. Review.
-
Olfactory marker protein contains a leucine-rich domain in the Ω-loop important for nuclear export.Mol Brain. 2022 Nov 4;15(1):89. doi: 10.1186/s13041-022-00973-0. Mol Brain. 2022. PMID: 36333725 Free PMC article.
References
-
- Boguslavsky RL, Stewart CL, Worman HJ. Nuclear lamin A inhibits adipocyte differentiation: implications for Dunnigan-type familial partial lipodystrophy. Hum Mol Genet 2006;15:653–663 - PubMed
-
- Oldenburg AR, Delbarre E, Thiede B, Vigouroux C, Collas P. Deregulation of Fragile X-related protein 1 by the lipodystrophic lamin A p.R482W mutation elicits a myogenic gene expression program in preadipocytes. Hum Mol Genet 2014;23:1151–1162 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- T32 DK071212/DK/NIDDK NIH HHS/United States
- R01 DK125513/DK/NIDDK NIH HHS/United States
- R24 DK084970/DK/NIDDK NIH HHS/United States
- F32 DK123887/DK/NIDDK NIH HHS/United States
- R01 AR044927/AR/NIAMS NIH HHS/United States
- F32 DK122654/DK/NIDDK NIH HHS/United States
- T32 DK101357/DK/NIDDK NIH HHS/United States
- T32 GM007863/GM/NIGMS NIH HHS/United States
- P30 AR069620/AR/NIAMS NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- R29 AR044927/AR/NIAMS NIH HHS/United States
- R01 DK121759/DK/NIDDK NIH HHS/United States
- U2C DK110768/DK/NIDDK NIH HHS/United States
- R01 AR065424/AR/NIAMS NIH HHS/United States
- R24 DK092759/DK/NIDDK NIH HHS/United States
- P30 DK089503/DK/NIDDK NIH HHS/United States
- T32 HD007505/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

