Safety and Immunogenicity of a Recombinant Tetanus Vaccine in Healthy Adults in China: A Randomized, Double-Blind, Dose Escalation, Placebo- and Positive-Controlled, Phase 1/2 Trial
- PMID: 34081408
- PMCID: PMC8336487
- DOI: 10.1002/advs.202002751
Safety and Immunogenicity of a Recombinant Tetanus Vaccine in Healthy Adults in China: A Randomized, Double-Blind, Dose Escalation, Placebo- and Positive-Controlled, Phase 1/2 Trial
Abstract
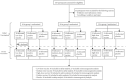
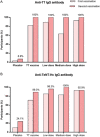
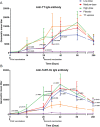
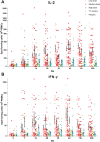
Tetanus is a fatal but vaccine-preventable disease. The currently available tetanus vaccines are tetanus toxoid (TT)-based. Although these vaccines are generally effective, challenges in vaccine development and access remain. A randomized, double-blind, dose escalation, placebo- and positive-controlled, phase 1/2 trial (ChiCTR1800015865) is performed to evaluate the safety and immunogenicity of an alternative recombinant tetanus vaccine based on the Hc domain of tetanus neurotoxin (TeNT-Hc) in healthy adult volunteers. The primary outcome is the safety profile of the recombinant tetanus vaccine, and immunogenicity is the secondary outcome. 150 eligible participants were enrolled and randomly assigned to receive one of the three doses of recombinant tetanus vaccine (TeNT-Hc 10/20/30 µg), TT vaccine, or placebo. The recombinant tetanus vaccine shows a good safety profile. The frequency of any solicited and unsolicited adverse events after each vaccination does not differ across the vaccine and placebo recipients. No serious treatment-related adverse events occur. The recombinant tetanus vaccine shows strong immune responses (seroconversion rates, geometric mean titer, and antigen-specific CD4+/CD8+ T-cell responses), which are roughly comparable to those of the TT vaccine. In conclusion, the findings from this study support that recombinant tetanus vaccine is safe and immunogenic; thereby, it represents a novel vaccine candidate against tetanus.
Keywords: recombinant tetanus vaccine; tetanus; tetanus toxoid vaccine.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Immunogenicity and safety of the new reduced-dose tetanus-diphtheria vaccine in healthy Korean adolescents: A comparative active control, double-blind, randomized, multicenter phase III study.J Microbiol Immunol Infect. 2017 Apr;50(2):207-213. doi: 10.1016/j.jmii.2015.04.005. Epub 2015 May 14. J Microbiol Immunol Infect. 2017. PMID: 26055693 Clinical Trial.
-
Safety and immunogenicity of a recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in Sierra Leone: a single-centre, randomised, double-blind, placebo-controlled, phase 2 trial.Lancet. 2017 Feb 11;389(10069):621-628. doi: 10.1016/S0140-6736(16)32617-4. Epub 2016 Dec 23. Lancet. 2017. PMID: 28017399 Clinical Trial.
-
Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials.Lancet Infect Dis. 2021 Aug;21(8):1107-1119. doi: 10.1016/S1473-3099(21)00127-4. Epub 2021 Mar 24. Lancet Infect Dis. 2021. PMID: 33773111 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of a single intramuscular dose of a tetanus-diphtheria toxoid (Td) vaccine (BR-TD-1001) in healthy Korean adult subjects.Hum Vaccin Immunother. 2015;11(10):2440-5. doi: 10.1080/21645515.2015.1054582. Hum Vaccin Immunother. 2015. PMID: 26091286 Free PMC article. Clinical Trial.
-
Meningococcal groups C and Y and haemophilus B tetanus toxoid conjugate vaccine (HibMenCY-TT; MenHibrix(®)): a review.Drugs. 2013 May;73(7):703-13. doi: 10.1007/s40265-013-0048-9. Drugs. 2013. PMID: 23649970 Review.
Cited by
-
A novel built-in adjuvant metallothionein-3 aids protein antigens to induce rapid, robust, and durable immune responses.Front Immunol. 2022 Nov 8;13:1024437. doi: 10.3389/fimmu.2022.1024437. eCollection 2022. Front Immunol. 2022. PMID: 36426348 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
