Erythrocyte Adhesion of Merozoite Surface Antigen 2c1 Expressed During Extracellular Stages of Babesia orientalis
- PMID: 34079537
- PMCID: PMC8165267
- DOI: 10.3389/fimmu.2021.623492
Erythrocyte Adhesion of Merozoite Surface Antigen 2c1 Expressed During Extracellular Stages of Babesia orientalis
Abstract
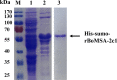
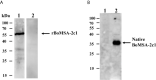
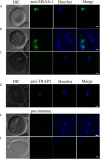
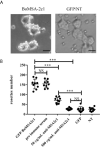
Babesia orientalis, a major infectious agent of water buffalo hemolytic babesiosis, is transmitted by Rhipicephalus haemaphysaloides. However, no effective vaccine is available. Essential antigens that are involved in parasite invasion of host red blood cells (RBCs) are potential vaccine candidates. Therefore, the identification and the conduction of functional studies of essential antigens are highly desirable. Here, we evaluated the function of B. orientalis merozoite surface antigen 2c1 (BoMSA-2c1), which belongs to the variable merozoite surface antigen (VMSA) family in B. orientalis. We developed a polyclonal antiserum against the purified recombinant (r)BoMSA-2c1 protein. Immunofluorescence staining results showed that BoMSA-2c1 was expressed only on extracellular merozoites, whereas the antigen was undetectable in intracellular parasites. RBC binding assays suggested that BoMSA-2c1 specifically bound to buffalo erythrocytes. Cytoadherence assays using a eukaryotic expression system in vitro further verified the binding and inhibitory ability of BoMSA-2c1. We found that BoMSA-2c1 with a GPI domain was expressed on the surface of HEK293T cells that bound to water buffalo RBCs, and that the anti-rBoMSA2c1 antibody inhibited this binding. These results indicated that BoMSA-2c1 was involved in mediating initial binding to host erythrocytes of B. orientalis. Identification of the occurrence of binding early in the invasion process may facilitate understanding of the growth characteristics, and may help in formulating strategies for the prevention and control of this parasite.
Keywords: Babesia orientalis; MSA-2c1; adhesion; invasion; merozoite surface antigen.
Copyright © 2021 Nie, Ao, Wang, Shu, Li, Zhan, Yu, An, Sun, Guo, Zhao, He and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Characterization of the variable merozoite surface antigen (VMSA) gene family of Babesia orientalis.Parasitol Res. 2020 Nov;119(11):3639-3648. doi: 10.1007/s00436-020-06877-z. Epub 2020 Sep 15. Parasitol Res. 2020. PMID: 32930858
-
Structural and functional characterization of Bc28.1, major erythrocyte-binding protein from Babesia canis merozoite surface.J Biol Chem. 2012 Mar 16;287(12):9495-508. doi: 10.1074/jbc.M111.260745. Epub 2012 Jan 31. J Biol Chem. 2012. PMID: 22294693 Free PMC article.
-
Identification of a novel variant erythrocyte surface antigen-1 (VESA1) in Babesia orientalis.Parasitol Res. 2021 Aug;120(8):2863-2872. doi: 10.1007/s00436-021-07194-9. Epub 2021 Jul 5. Parasitol Res. 2021. PMID: 34219188 Free PMC article.
-
Genetic basis for GPI-anchor merozoite surface antigen polymorphism of Babesia and resulting antigenic diversity.Vet Parasitol. 2006 May 31;138(1-2):33-49. doi: 10.1016/j.vetpar.2006.01.038. Epub 2006 Mar 23. Vet Parasitol. 2006. PMID: 16551492 Review.
-
Erythrocyte invasion by Babesia parasites: current advances in the elucidation of the molecular interactions between the protozoan ligands and host receptors in the invasion stage.Vet Parasitol. 2006 May 31;138(1-2):22-32. doi: 10.1016/j.vetpar.2006.01.037. Epub 2006 Feb 28. Vet Parasitol. 2006. PMID: 16504403 Review.
Cited by
-
Characterization of anti-erythrocyte and anti-platelet antibodies in hemolytic anemia and thrombocytopenia induced by Plasmodium spp. and Babesiaspp. infection in mice.Front Cell Infect Microbiol. 2023 Apr 14;13:1143138. doi: 10.3389/fcimb.2023.1143138. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37124034 Free PMC article.
-
Advances in Babesia Vaccine Development: An Overview.Pathogens. 2023 Feb 11;12(2):300. doi: 10.3390/pathogens12020300. Pathogens. 2023. PMID: 36839572 Free PMC article. Review.
References
-
- Zimmer AJ, Simonsen KA. Babesiosis. In: Statpearls. Treasure Island (FL: StatPearls Publishing LLC; (2021). StatPearls Publishing 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

