Accuracy of malaria diagnostic tests performed on non-invasively collected samples: a systematic review and meta-analysis
- PMID: 34078631
- PMCID: PMC8173286
- DOI: 10.1136/bmjgh-2021-005634
Accuracy of malaria diagnostic tests performed on non-invasively collected samples: a systematic review and meta-analysis
Abstract
Background: During the last decade, many studies have assessed the performance of malaria tests on non-invasively collected specimens, but no systematic review has hitherto estimated the overall performance of these tests. We report here the first meta-analysis estimating the diagnostic performance of malaria diagnostic tests performed on saliva, urine, faeces, skin odour ('sniff and tell') and hair, using either microscopy or PCR on blood sample as reference test.
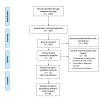
Methods: We searched on PubMed, EMBASE, African Journals Online and Cochrane Infectious Diseases from inception until 19 January 2021 for relevant primary studies. A random effects model was used to estimate the overall performance of various diagnostic methods on different types of specimen.
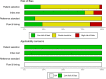
Results: Eighteen studies providing 30 data sets were included in the meta-analysis. The overall sensitivity, specificity and diagnostic OR (DOR) of PCR were 84.5% (95% CI 79.3% to 88.6%), 97.3% (95% CI 95.3% to 98.5%) and 184.9 (95% CI 95.8 to 356.9) in saliva, respectively; 57.4% (95% CI 41.4% to 72.1%), 98.6% (95% CI 97.3% to 99.3%) and 47.2 (95% CI 22.1 to 101.1) in urine, respectively. The overall sensitivity, specificity and DOR of rapid diagnostic test for malaria in urine was 59.8% (95% CI 40.0% to 76.9%), 96.9% (95% CI 91.0% to 99.0%) and 30.8 (95% CI:23.5 to 40.4).
Conclusion: In settings where PCR is available, saliva and urine samples should be considered for PCR-based malaria diagnosis only if blood samples cannot be collected. The performance of rapid diagnostic testing in the urine is limited, especially its sensitivity. Malaria testing on non-invasively collected specimen still needs substantial improvement.
Keywords: malaria; public Health.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Systematic review and meta-analysis of diagnostic accuracy of loop-mediated isothermal amplification (LAMP) methods compared with microscopy, polymerase chain reaction and rapid diagnostic tests for malaria diagnosis.Int J Infect Dis. 2020 Sep;98:408-419. doi: 10.1016/j.ijid.2020.07.009. Epub 2020 Jul 11. Int J Infect Dis. 2020. PMID: 32659450
-
Comparison of diagnostic performance between conventional and ultrasensitive rapid diagnostic tests for diagnosis of malaria: A systematic review and meta-analysis.PLoS One. 2022 Feb 10;17(2):e0263770. doi: 10.1371/journal.pone.0263770. eCollection 2022. PLoS One. 2022. PMID: 35143565 Free PMC article.
-
Performance of rapid diagnostic test, blood-film microscopy and PCR for the diagnosis of malaria infection among febrile children from Korogwe District, Tanzania.Malar J. 2016 Jul 26;15(1):391. doi: 10.1186/s12936-016-1450-z. Malar J. 2016. PMID: 27459856 Free PMC article.
-
Urine and Saliva: Relevant Specimens for Malaria Diagnosis?Diagnostics (Basel). 2022 Nov 29;12(12):2989. doi: 10.3390/diagnostics12122989. Diagnostics (Basel). 2022. PMID: 36552996 Free PMC article. Review.
-
Polymerase chain reaction blood tests for the diagnosis of invasive aspergillosis in immunocompromised people.Cochrane Database Syst Rev. 2015 Oct 1;(10):CD009551. doi: 10.1002/14651858.CD009551.pub3. Cochrane Database Syst Rev. 2015. PMID: 26424726 Review.
Cited by
-
Limited Reliability of the Molecular Detection of Plasmodium spp. from Incubated Blood Culture Samples for Forensic Purposes.Microorganisms. 2022 Feb 10;10(2):406. doi: 10.3390/microorganisms10020406. Microorganisms. 2022. PMID: 35208861 Free PMC article.
-
Acceptance and perceived value of non-invasive malaria diagnostic tests in malaria-endemic countries.Malar J. 2021 Sep 24;20(1):379. doi: 10.1186/s12936-021-03911-y. Malar J. 2021. PMID: 34560899 Free PMC article.
References
-
- World malaria report 2020 [online]. Available: https://www.who.int/teams/global-malaria-programme/reports/world-malaria... [Accessed 23 Dec 2020].
-
- World Health Organization . Global malaria programme. global technical strategy for malaria, 2016-2030, 2015.
-
- World Health Organization . T3: test. treat. track. Scaling up diagnostic testing, treatment and surveillance for malaria [online]. Available: https://www.who.int/malaria/publications/atoz/t3_brochure/en/ [Accessed 23 Dec 2020].
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical