The roles of tumor-derived exosomes in altered differentiation, maturation and function of dendritic cells
- PMID: 34078376
- PMCID: PMC8170799
- DOI: 10.1186/s12943-021-01376-w
The roles of tumor-derived exosomes in altered differentiation, maturation and function of dendritic cells
Abstract
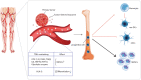
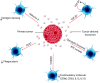
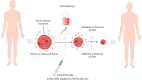
Tumor-derived exosomes (TDEs) have been shown to impede anti-tumor immune responses via their immunosuppressive cargo. Since dendritic cells (DCs) are the key mediators of priming and maintenance of T cell-mediated responses; thus it is logical that the exosomes released by tumor cells can exert a dominant influence on DCs biology. This paper intends to provide a mechanistic insight into the TDEs-mediated DCs abnormalities in the tumor context. More importantly, we discuss extensively how tumor exosomes induce subversion of DCs differentiation, maturation and function in separate sections. We also briefly describe the importance of TDEs at therapeutic level to help guide future treatment options, in particular DC-based vaccination strategy, and review advances in the design and discovery of exosome inhibitors. Understanding the exosomal content and the pathways by which TDEs are responsible for immune evasion may help to revise treatment rationales and devise novel therapeutic approaches to overcome the hurdles in cancer treatment.
Keywords: Dendritic cell; Exosome; Immunity; Tumor.
Conflict of interest statement
The authors declare that they have no competing of interests.
Figures

Similar articles
-
Tumor exosomes block dendritic cells maturation to decrease the T cell immune response.Immunol Lett. 2018 Jul;199:36-43. doi: 10.1016/j.imlet.2018.05.002. Epub 2018 May 22. Immunol Lett. 2018. PMID: 29800589
-
Role of tumor-derived exosomes mediated immune cell reprograming in cancer.Gene. 2024 Oct 20;925:148601. doi: 10.1016/j.gene.2024.148601. Epub 2024 May 23. Gene. 2024. PMID: 38788817 Review.
-
In vitro and ex vivo evaluation of tumor-derived exosome-induced dendritic cell dysfunction in mouse.STAR Protoc. 2021 Mar 4;2(1):100361. doi: 10.1016/j.xpro.2021.100361. eCollection 2021 Mar 19. STAR Protoc. 2021. PMID: 33786458 Free PMC article.
-
Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment.Front Immunol. 2018 Dec 20;9:3059. doi: 10.3389/fimmu.2018.03059. eCollection 2018. Front Immunol. 2018. PMID: 30619378 Free PMC article. Review.
-
Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models.J Hepatol. 2017 Oct;67(4):739-748. doi: 10.1016/j.jhep.2017.05.019. Epub 2017 May 24. J Hepatol. 2017. PMID: 28549917
Cited by
-
Therapeutic combinations of exosomes alongside cancer stem cells (CSCs) and of CSC-derived exosomes (CSCEXs) in cancer therapy.Cancer Cell Int. 2024 Oct 5;24(1):334. doi: 10.1186/s12935-024-03514-y. Cancer Cell Int. 2024. PMID: 39369258 Free PMC article. Review.
-
Cancer exosomes and natural killer cells dysfunction: biological roles, clinical significance and implications for immunotherapy.Mol Cancer. 2022 Jan 14;21(1):15. doi: 10.1186/s12943-021-01492-7. Mol Cancer. 2022. PMID: 35031075 Free PMC article. Review.
-
Research Progress of Dendritic Cell Surface Receptors and Targeting.Biomedicines. 2023 Jun 9;11(6):1673. doi: 10.3390/biomedicines11061673. Biomedicines. 2023. PMID: 37371768 Free PMC article. Review.
-
Placental homing peptide guides HIF1α‑silenced exosomes conjugates for targeted enhancement of invasion of trophoblast cells.Mol Med Rep. 2023 Jul;28(1):135. doi: 10.3892/mmr.2023.13022. Epub 2023 May 26. Mol Med Rep. 2023. PMID: 37232338 Free PMC article.
-
Roles of exosomes in immunotherapy for solid cancers.Cell Death Dis. 2024 Feb 1;15(2):106. doi: 10.1038/s41419-024-06494-z. Cell Death Dis. 2024. PMID: 38302430 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

