Mitochondrial Reactive Oxygen Species Enhance Alveolar Macrophage Activity against Aspergillus fumigatus but Are Dispensable for Host Protection
- PMID: 34077261
- PMCID: PMC8265640
- DOI: 10.1128/mSphere.00260-21
Mitochondrial Reactive Oxygen Species Enhance Alveolar Macrophage Activity against Aspergillus fumigatus but Are Dispensable for Host Protection
Abstract
Aspergillus fumigatus is the most common cause of mold pneumonia worldwide, and a significant cause of infectious morbidity and mortality in immunocompromised individuals. The oxidative burst, which generates reactive oxidative species (ROS), plays a pivotal role in host defense against aspergillosis and induces regulated cell death in Aspergillus conidia, the infectious propagules. Beyond the well-established role of NADP (NADPH) oxidase in ROS generation by neutrophils and other innate effector cells, mitochondria represent a major ROS production site in many cell types, though it is unclear whether mitochondrial ROS (mtROS) contribute to antifungal activity in the lung. Following A. fumigatus infection, we observed that innate effector cells, including alveolar macrophages (AMs), monocyte-derived dendritic cells (Mo-DCS), and neutrophils, generated mtROS, primarily in fungus-infected cells. To examine the functional role of mtROS, specifically the H2O2 component, in pulmonary host defense against A. fumigatus, we infected transgenic mice that expressed a mitochondrion-targeted catalase. Using a reporter of fungal viability during interactions with leukocytes, mitochondrial H2O2 (mtH2O2) was essential for optimal AM, but not for neutrophil phagocytic and conidiacidal activity in the lung. Catalase-mediated mtH2O2 neutralization did not lead to invasive aspergillosis in otherwise immunocompetent mice and did not shorten survival in mice that lack NADPH oxidase function. Collectively, these studies indicate that mtROS-associated defects in AM antifungal activity can be functionally compensated by the action of NADPH oxidase and by nonoxidative effector mechanisms during murine A. fumigatus lung infection. IMPORTANCE Aspergillus fumigatus is a fungal pathogen that causes invasive disease in humans with defects in immune function. Airborne conidia, the infectious propagules, are ubiquitous and inhaled on a daily basis. In the respiratory tree, conidia are killed by the coordinated actions of phagocytes, including alveolar macrophages, neutrophils, and monocyte-derived dendritic cells. The oxidative burst represents a central killing mechanism and relies on the assembly of the NADPH oxidase complex on the phagosomal membrane. However, NADPH oxidase-deficient leukocytes have significant residual fungicidal activity in vivo, indicating the presence of alternative effector mechanisms. Here, we report that murine innate immune cells produce mitochondrial reactive oxygen species (mtROS) in response to fungal interactions. Neutralizing the mtROS constituent hydrogen peroxide (H2O2) via a catalase expressed in mitochondria of innate immune cells substantially diminished fungicidal properties of alveolar macrophages, but not of other innate immune cells. These data indicate that mtH2O2 represent a novel AM killing mechanism against Aspergillus conidia. mtH2O2 neutralization is compensated by other killing mechanisms in the lung, demonstrating functional redundancy at the level of host defense in the respiratory tree. These findings have important implications for the development of host-directed therapies against invasive aspergillosis in susceptible patient populations.
Keywords: fungus; innate immunity; lung.
Figures
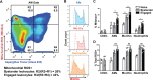

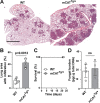
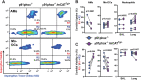
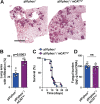
Similar articles
-
During Aspergillus Infection, Monocyte-Derived DCs, Neutrophils, and Plasmacytoid DCs Enhance Innate Immune Defense through CXCR3-Dependent Crosstalk.Cell Host Microbe. 2020 Jul 8;28(1):104-116.e4. doi: 10.1016/j.chom.2020.05.002. Epub 2020 Jun 1. Cell Host Microbe. 2020. PMID: 32485165 Free PMC article.
-
Three-Dimensional Light Sheet Fluorescence Microscopy of Lungs To Dissect Local Host Immune-Aspergillus fumigatus Interactions.mBio. 2020 Feb 4;11(1):e02752-19. doi: 10.1128/mBio.02752-19. mBio. 2020. PMID: 32019790 Free PMC article.
-
Mitochondrial Reactive Oxygen Species Regulate Immune Responses of Macrophages to Aspergillus fumigatus.Front Immunol. 2021 Mar 25;12:641495. doi: 10.3389/fimmu.2021.641495. eCollection 2021. Front Immunol. 2021. PMID: 33841423 Free PMC article.
-
Pulmonary defense mechanisms against opportunistic fungal pathogens.Immunol Ser. 1989;47:243-71. Immunol Ser. 1989. PMID: 2490078 Review.
-
New developments in Aspergillus fumigatus and host reactive oxygen species responses.Curr Opin Microbiol. 2024 Aug;80:102521. doi: 10.1016/j.mib.2024.102521. Epub 2024 Jul 29. Curr Opin Microbiol. 2024. PMID: 39079399 Review.
Cited by
-
Perforin-2 is dispensable for host defense against Aspergillus fumigatus and Candida albicans.bioRxiv [Preprint]. 2024 Sep 25:2024.09.23.614582. doi: 10.1101/2024.09.23.614582. bioRxiv. 2024. Update in: mSphere. 2025 Jan 28;10(1):e0080324. doi: 10.1128/msphere.00803-24. PMID: 39386632 Free PMC article. Updated. Preprint.
-
A Fun-Guide to Innate Immune Responses to Fungal Infections.J Fungi (Basel). 2022 Jul 29;8(8):805. doi: 10.3390/jof8080805. J Fungi (Basel). 2022. PMID: 36012793 Free PMC article. Review.
-
Neutrophil and Macrophage NADPH Oxidase 2 Differentially Control Responses to Inflammation and to Aspergillus fumigatus in Mice.J Immunol. 2022 Nov 15;209(10):1960-1972. doi: 10.4049/jimmunol.2200543. J Immunol. 2022. PMID: 36426951 Free PMC article.
-
Respiratory Epithelial Cells: More Than Just a Physical Barrier to Fungal Infections.J Fungi (Basel). 2022 May 24;8(6):548. doi: 10.3390/jof8060548. J Fungi (Basel). 2022. PMID: 35736031 Free PMC article. Review.
-
Mitochondrial Reactive Oxygen Species in Infection and Immunity.Biomolecules. 2024 Jun 8;14(6):670. doi: 10.3390/biom14060670. Biomolecules. 2024. PMID: 38927073 Free PMC article. Review.
References
-
- Paulussen C, Hallsworth JE, Álvarez-Pérez S, Nierman WC, Hamill PG, Blain D, Rediers H, Lievens B. 2017. Ecology of aspergillosis: insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microb Biotechnol 10:296–322. doi:10.1111/1751-7915.12367. - DOI - PMC - PubMed
-
- Guo Y, Kasahara S, Jhingran A, Tosini NL, Zhai B, Aufiero MA, Mills KAM, Gjonbalaj M, Espinosa V, Rivera A, Luster AD, Hohl TM. 2020. During Aspergillus infection, monocyte-derived DCs, neutrophils, and plasmacytoid DCs enhance innate immune defense through CXCR3-dependent crosstalk. Cell Host Microbe 28:104–116.e104. doi:10.1016/j.chom.2020.05.002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
