Age-Related Alterations at Neuromuscular Junction: Role of Oxidative Stress and Epigenetic Modifications
- PMID: 34074012
- PMCID: PMC8225025
- DOI: 10.3390/cells10061307
Age-Related Alterations at Neuromuscular Junction: Role of Oxidative Stress and Epigenetic Modifications
Abstract
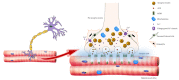
With advancing aging, a decline in physical abilities occurs, leading to reduced mobility and loss of independence. Although many factors contribute to the physio-pathological effects of aging, an important event seems to be related to the compromised integrity of the neuromuscular system, which connects the brain and skeletal muscles via motoneurons and the neuromuscular junctions (NMJs). NMJs undergo severe functional, morphological, and molecular alterations during aging and ultimately degenerate. The effect of this decline is an inexorable decrease in skeletal muscle mass and strength, a condition generally known as sarcopenia. Moreover, several studies have highlighted how the age-related alteration of reactive oxygen species (ROS) homeostasis can contribute to changes in the neuromuscular junction morphology and stability, leading to the reduction in fiber number and innervation. Increasing evidence supports the involvement of epigenetic modifications in age-dependent alterations of the NMJ. In particular, DNA methylation, histone modifications, and miRNA-dependent gene expression represent the major epigenetic mechanisms that play a crucial role in NMJ remodeling. It is established that environmental and lifestyle factors, such as physical exercise and nutrition that are susceptible to change during aging, can modulate epigenetic phenomena and attenuate the age-related NMJs changes. This review aims to highlight the recent epigenetic findings related to the NMJ dysregulation during aging and the role of physical activity and nutrition as possible interventions to attenuate or delay the age-related decline in the neuromuscular system.
Keywords: ALS; ROS; aging; exercise; nutrition; oxidative stress; skeletal muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Muscle mitochondrial catalase expression prevents neuromuscular junction disruption, atrophy, and weakness in a mouse model of accelerated sarcopenia.J Cachexia Sarcopenia Muscle. 2021 Dec;12(6):1582-1596. doi: 10.1002/jcsm.12768. Epub 2021 Sep 24. J Cachexia Sarcopenia Muscle. 2021. PMID: 34559475 Free PMC article.
-
VAChT overexpression increases acetylcholine at the synaptic cleft and accelerates aging of neuromuscular junctions.Skelet Muscle. 2016 Oct 5;6:31. doi: 10.1186/s13395-016-0105-7. eCollection 2016. Skelet Muscle. 2016. PMID: 27713817 Free PMC article.
-
A Role of Lamin A/C in Preventing Neuromuscular Junction Decline in Mice.J Neurosci. 2020 Sep 16;40(38):7203-7215. doi: 10.1523/JNEUROSCI.0443-20.2020. Epub 2020 Aug 17. J Neurosci. 2020. PMID: 32817327 Free PMC article.
-
Neuromuscular junction transmission failure in aging and sarcopenia: The nexus of the neurological and muscular systems.Ageing Res Rev. 2023 Aug;89:101966. doi: 10.1016/j.arr.2023.101966. Epub 2023 Jun 1. Ageing Res Rev. 2023. PMID: 37270145 Free PMC article. Review.
-
Neuromuscular Junction Aging: A Role for Biomarkers and Exercise.J Gerontol A Biol Sci Med Sci. 2021 Mar 31;76(4):576-585. doi: 10.1093/gerona/glaa207. J Gerontol A Biol Sci Med Sci. 2021. PMID: 32832976 Review.
Cited by
-
Unraveling the causes of sarcopenia: Roles of neuromuscular junction impairment and mitochondrial dysfunction.Physiol Rep. 2024 Jan;12(1):e15917. doi: 10.14814/phy2.15917. Physiol Rep. 2024. PMID: 38225199 Free PMC article. Review.
-
A posteriori dietary patterns in 71-year-old Swedish men and the prevalence of sarcopenia 16 years later.Br J Nutr. 2021 Sep 29;128(5):1-12. doi: 10.1017/S0007114521003901. Online ahead of print. Br J Nutr. 2021. PMID: 34585650 Free PMC article.
-
Macrophage Involvement in Aging-Associated Skeletal Muscle Regeneration.Cells. 2023 Apr 22;12(9):1214. doi: 10.3390/cells12091214. Cells. 2023. PMID: 37174614 Free PMC article. Review.
-
Leveraging Biomaterial Platforms to Study Aging-Related Neural and Muscular Degeneration.Biomolecules. 2024 Jan 4;14(1):69. doi: 10.3390/biom14010069. Biomolecules. 2024. PMID: 38254669 Free PMC article. Review.
-
A combination of metformin and galantamine exhibits synergistic benefits in the treatment of sarcopenia.JCI Insight. 2023 Aug 8;8(15):e168787. doi: 10.1172/jci.insight.168787. JCI Insight. 2023. PMID: 37551712 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

