Refinement of an Established Procedure and Its Application for Identification of Hypoxia in Prostate Cancer Xenografts
- PMID: 34073301
- PMCID: PMC8198481
- DOI: 10.3390/cancers13112602
Refinement of an Established Procedure and Its Application for Identification of Hypoxia in Prostate Cancer Xenografts
Abstract
Background: This pre-clinical study was designed to refine a dissection method for validating the use of a 15-gene hypoxia classifier, which was previously established for head and neck squamous cell carcinoma (HNSCC) patients, to identify hypoxia in prostate cancer.
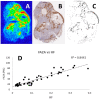
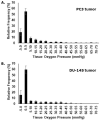
Methods: PC3 and DU-145 adenocarcinoma cells, in vitro, were gassed with various oxygen concentrations (0-21%) for 24 h, followed by real-time PCR. Xenografts were established in vivo, and the mice were injected with the hypoxic markers [18F]-FAZA and pimonidazole. Subsequently, tumors were excised, frozen, cryo-sectioned, and analyzed using autoradiography ([18F]-FAZA) and immunohistochemistry (pimonidazole); the autoradiograms used as templates for laser capture microdissection of hypoxic and non-hypoxic areas, which were lysed, and real-time PCR was performed.
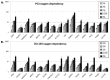
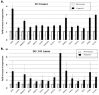
Results: In vitro, all 15 genes were increasingly up-regulated as oxygen concentrations decreased. With the xenografts, all 15 genes were up-regulated in the hypoxic compared to non-hypoxic areas for both cell lines, although this effect was greater in the DU-145.
Conclusions: We have developed a combined autoradiographic/laser-guided microdissection method with broad applicability. Using this approach on fresh frozen tumor material, thereby minimizing the degree of RNA degradation, we showed that the 15-gene hypoxia gene classifier developed in HNSCC may be applicable for adenocarcinomas such as prostate cancer.
Keywords: DU-145; PC3; hypoxia; hypoxia gene signature; pre-clinical models; prostate cancer.
Conflict of interest statement
B.S. Sørensen is a co-inventor on a patent on a method (gene expression profile) for determining clinically relevant hypoxia in cancer (WO2012146259A1). All other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
The usability of a 15-gene hypoxia classifier as a universal hypoxia profile in various cancer cell types.Radiother Oncol. 2015 Sep;116(3):346-51. doi: 10.1016/j.radonc.2015.06.028. Epub 2015 Jul 10. Radiother Oncol. 2015. PMID: 26169282
-
Correlation of [18F]FMISO autoradiography and pimonidazole [corrected] immunohistochemistry in human head and neck carcinoma xenografts.Eur J Nucl Med Mol Imaging. 2008 Oct;35(10):1803-11. doi: 10.1007/s00259-008-0772-7. Epub 2008 Apr 18. Eur J Nucl Med Mol Imaging. 2008. PMID: 18421457
-
Influence of FAZA PET hypoxia and HPV-status for the outcome of head and neck squamous cell carcinoma (HNSCC) treated with radiotherapy: Long-term results from the DAHANCA 24 trial (NCT01017224).Radiother Oncol. 2020 Oct;151:126-133. doi: 10.1016/j.radonc.2020.08.006. Epub 2020 Aug 14. Radiother Oncol. 2020. PMID: 32805273
-
1-(5-[18F]Fluoro-5-deoxy-α-D-arabinofuranosyl)-2-nitroimidazole.2005 Jul 19 [updated 2009 Dec 27]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2005 Jul 19 [updated 2009 Dec 27]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641484 Free Books & Documents. Review.
-
Longitudinal PET imaging of tumor hypoxia during the course of radiotherapy.Eur J Nucl Med Mol Imaging. 2018 Nov;45(12):2201-2217. doi: 10.1007/s00259-018-4116-y. Epub 2018 Aug 20. Eur J Nucl Med Mol Imaging. 2018. PMID: 30128659 Review.
Cited by
-
Accurate Three-Dimensional Thermal Dosimetry and Assessment of Physiologic Response Are Essential for Optimizing Thermoradiotherapy.Cancers (Basel). 2022 Mar 27;14(7):1701. doi: 10.3390/cancers14071701. Cancers (Basel). 2022. PMID: 35406473 Free PMC article. Review.
-
Clinicopathological Significance of Defective DNA Mismatch Repair in Endometrial Carcinoma: A Single-Center Study From Bahrain.Cureus. 2024 Aug 20;16(8):e67332. doi: 10.7759/cureus.67332. eCollection 2024 Aug. Cureus. 2024. PMID: 39301379 Free PMC article.
References
-
- Vaupel P., Kallinowski F., Okunieff P. Blood Flow, Oxygen and Nutrient Supply, and Metabolic Microenvironment of Human Tumors: A Review. Cancer Res. 1989;49:6449–6465. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

