Botrytis cinerea G Protein β Subunit Bcgb1 Controls Growth, Development and Virulence by Regulating cAMP Signaling and MAPK Signaling
- PMID: 34072395
- PMCID: PMC8228952
- DOI: 10.3390/jof7060431
Botrytis cinerea G Protein β Subunit Bcgb1 Controls Growth, Development and Virulence by Regulating cAMP Signaling and MAPK Signaling
Abstract
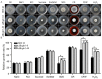
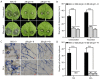
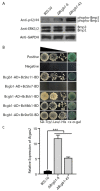
Botrytis cinerea is a necrotrophic phytopathogenic fungus that causes gray mold disease in many crops. To better understand the role of G protein signaling in the development and virulence of this fungus, the G protein β subunit gene Bcgb1 was knocked out in this study. The ΔBcgb1 mutants showed reduced mycelial growth rate, but increased aerial hyphae and mycelial biomass, lack of conidiation, failed to form sclerotia, increased resistance to cell wall and oxidative stresses, delayed formation of infection cushions, and decreased virulence. Deletion of Bcgb1 resulted in a significant reduction in the expression of several genes involved in cAMP signaling, and caused a notable increase in intracellular cAMP levels, suggesting that G protein β subunit Bcgb1 plays an important role in cAMP signaling. Furthermore, phosphorylation levels of MAP kinases (Bmp1 and Bmp3) were increased in the ΔBcgb1 mutants. Yeast two-hybrid assays showed that Bcgb1 interacts with MAPK (Bmp1 and Bmp3) cascade proteins (BcSte11, BcBck1, BcMkk1, and BcSte50), and the Bmp1-regulated gene Bcgas2 was up-regulated in the ΔBcgb1 mutant. These results indicated that Gβ protein Bcgb1 is involved in the MAPK signaling pathway in B. cinerea. In summary, our results revealed that Gβ protein Bcgb1 controls development and virulence through both the cAMP and MAPK (Bmp1 and Bmp3) signaling pathways in B. cinerea.
Keywords: Botrytis cinerea; Gβ subunit; MAPK signaling pathway; cAMP signaling pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


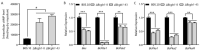

Similar articles
-
The Gβ-like protein Bcgbl1 regulates development and pathogenicity of the gray mold Botrytis cinerea via modulating two MAP kinase signaling pathways.PLoS Pathog. 2023 Dec 4;19(12):e1011839. doi: 10.1371/journal.ppat.1011839. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38048363 Free PMC article.
-
The MAPK kinase BcMkk1 suppresses oxalic acid biosynthesis via impeding phosphorylation of BcRim15 by BcSch9 in Botrytis cinerea.PLoS Pathog. 2018 Sep 13;14(9):e1007285. doi: 10.1371/journal.ppat.1007285. eCollection 2018 Sep. PLoS Pathog. 2018. PMID: 30212570 Free PMC article.
-
The signalling mucin Msb2 regulates surface sensing and host penetration via BMP1 MAP kinase signalling in Botrytis cinerea.Mol Plant Pathol. 2015 Oct;16(8):787-98. doi: 10.1111/mpp.12234. Epub 2015 Feb 27. Mol Plant Pathol. 2015. PMID: 25582910 Free PMC article.
-
The BMP1 gene is essential for pathogenicity in the gray mold fungus Botrytis cinerea.Mol Plant Microbe Interact. 2000 Jul;13(7):724-32. doi: 10.1094/MPMI.2000.13.7.724. Mol Plant Microbe Interact. 2000. PMID: 10875333
-
Recent Advances in the Study of the Plant Pathogenic Fungus Botrytis cinerea and its Interaction with the Environment.Curr Protein Pept Sci. 2017;18(10):976-989. doi: 10.2174/1389203717666160809160915. Curr Protein Pept Sci. 2017. PMID: 27526927 Review.
Cited by
-
Cross-Talk and Multiple Control of Target of Rapamycin (TOR) in Sclerotinia sclerotiorum.Microbiol Spectr. 2023 Mar 21;11(2):e0001323. doi: 10.1128/spectrum.00013-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 36943069 Free PMC article.
-
The Gβ-like protein Bcgbl1 regulates development and pathogenicity of the gray mold Botrytis cinerea via modulating two MAP kinase signaling pathways.PLoS Pathog. 2023 Dec 4;19(12):e1011839. doi: 10.1371/journal.ppat.1011839. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38048363 Free PMC article.
-
Special Issue "Signal Transductions in Fungi".J Fungi (Basel). 2022 May 20;8(5):528. doi: 10.3390/jof8050528. J Fungi (Basel). 2022. PMID: 35628783 Free PMC article.
-
Transcriptome Analysis Revealed the Mechanism of Inhibition of Saprophytic Growth of Sparassis latifolia by Excessive Oxalic Acid.Cells. 2022 Nov 16;11(22):3636. doi: 10.3390/cells11223636. Cells. 2022. PMID: 36429064 Free PMC article.
-
A novel zinc finger transcription factor, BcMsn2, is involved in growth, development, and virulence in Botrytis cinerea.Front Microbiol. 2023 Oct 17;14:1247072. doi: 10.3389/fmicb.2023.1247072. eCollection 2023. Front Microbiol. 2023. PMID: 37915851 Free PMC article.
References
-
- Elad Y., Pertot I., Prado A.M.C., Stewart A. Plant hosts of Botrytis spp. In: Fillinger S., Elad Y., editors. Botrytis-the Fungus, the Pathogen and Its Management in Agricultural Systems. Springer; Berlin/Heidelberg, Germany: 2016. p. 6.
-
- Carisse O. Epidemiology and aerobiology of Botryris spp. In: Fillinger S., Elad Y., editors. Botrytis-the Fungus, the Pathogen and Its Management in Agricultural Systems. Springer; Berlin/Heidelberg, Germany: 2016. p. 133.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

