A Comprehensive Review: Sphingolipid Metabolism and Implications of Disruption in Sphingolipid Homeostasis
- PMID: 34071409
- PMCID: PMC8198874
- DOI: 10.3390/ijms22115793
A Comprehensive Review: Sphingolipid Metabolism and Implications of Disruption in Sphingolipid Homeostasis
Abstract
Sphingolipids are a specialized group of lipids essential to the composition of the plasma membrane of many cell types; however, they are primarily localized within the nervous system. The amphipathic properties of sphingolipids enable their participation in a variety of intricate metabolic pathways. Sphingoid bases are the building blocks for all sphingolipid derivatives, comprising a complex class of lipids. The biosynthesis and catabolism of these lipids play an integral role in small- and large-scale body functions, including participation in membrane domains and signalling; cell proliferation, death, migration, and invasiveness; inflammation; and central nervous system development. Recently, sphingolipids have become the focus of several fields of research in the medical and biological sciences, as these bioactive lipids have been identified as potent signalling and messenger molecules. Sphingolipids are now being exploited as therapeutic targets for several pathologies. Here we present a comprehensive review of the structure and metabolism of sphingolipids and their many functional roles within the cell. In addition, we highlight the role of sphingolipids in several pathologies, including inflammatory disease, cystic fibrosis, cancer, Alzheimer's and Parkinson's disease, and lysosomal storage disorders.
Keywords: biosynthesis; ceramide; glycosphingolipids; glycosyl hydrolase; inflammation; lysosomal storage disorder; neurodegeneration; sphingolipid; sphingosine-1-phosphate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
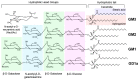
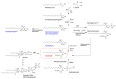

Similar articles
-
Role of bioactive sphingolipids in physiology and pathology.Essays Biochem. 2020 Sep 23;64(3):579-589. doi: 10.1042/EBC20190091. Essays Biochem. 2020. PMID: 32579188 Review.
-
An overview of sphingolipid metabolism: from synthesis to breakdown.Adv Exp Med Biol. 2010;688:1-23. doi: 10.1007/978-1-4419-6741-1_1. Adv Exp Med Biol. 2010. PMID: 20919643 Free PMC article. Review.
-
Sphingolipids--the enigmatic lipid class: biochemistry, physiology, and pathophysiology.Toxicol Appl Pharmacol. 1997 Jan;142(1):208-25. doi: 10.1006/taap.1996.8029. Toxicol Appl Pharmacol. 1997. PMID: 9007051 Review.
-
Novelty of Sphingolipids in the Central Nervous System Physiology and Disease: Focusing on the Sphingolipid Hypothesis of Neuroinflammation and Neurodegeneration.Int J Mol Sci. 2021 Jul 8;22(14):7353. doi: 10.3390/ijms22147353. Int J Mol Sci. 2021. PMID: 34298977 Free PMC article. Review.
-
Principles of bioactive lipid signalling: lessons from sphingolipids.Nat Rev Mol Cell Biol. 2008 Feb;9(2):139-50. doi: 10.1038/nrm2329. Nat Rev Mol Cell Biol. 2008. PMID: 18216770 Review.
Cited by
-
Lipids in Equine Airway Inflammation: An Overview of Current Knowledge.Animals (Basel). 2024 Jun 18;14(12):1812. doi: 10.3390/ani14121812. Animals (Basel). 2024. PMID: 38929431 Free PMC article. Review.
-
An update on genetically encoded lipid biosensors.Mol Biol Cell. 2022 May 1;33(5):tp2. doi: 10.1091/mbc.E21-07-0363. Mol Biol Cell. 2022. PMID: 35420888 Free PMC article.
-
Inulin and Chinese Gallotannin Affect Meat Quality and Lipid Metabolism on Hu Sheep.Animals (Basel). 2022 Dec 31;13(1):160. doi: 10.3390/ani13010160. Animals (Basel). 2022. PMID: 36611769 Free PMC article.
-
Ceramide/Sphingosine 1-Phosphate Axis as a Key Target for Diagnosis and Treatment in Alzheimer's Disease and Other Neurodegenerative Diseases.Int J Mol Sci. 2022 Jul 22;23(15):8082. doi: 10.3390/ijms23158082. Int J Mol Sci. 2022. PMID: 35897658 Free PMC article. Review.
-
What should rheumatologists know about Gaucher disease and Fabry disease? Connecting the dots for an overview.Adv Rheumatol. 2024 Mar 22;64(1):22. doi: 10.1186/s42358-024-00362-2. Adv Rheumatol. 2024. PMID: 38520029 Review.
References
-
- Thudichum J.L.W. A Treatise on the Chemical Constitution of the Brain: Based Throughout upon Original Researches. Glasg. Med. J. 1884;22:363–364.
-
- Schnaar R.L., Kinoshita T. Glycosphingolipids. In: Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Darvill A.G., Kinoshita T., Packer N.H., Prestegard J.H., Schnaar R.L., Seeberger P.H., editors. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2015. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

