Regional Differences in Heat Shock Protein 25 Expression in Brain and Spinal Cord Astrocytes of Wild-Type and SOD1 G93A Mice
- PMID: 34069691
- PMCID: PMC8160835
- DOI: 10.3390/cells10051257
Regional Differences in Heat Shock Protein 25 Expression in Brain and Spinal Cord Astrocytes of Wild-Type and SOD1 G93A Mice
Abstract
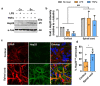
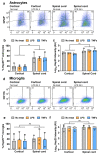
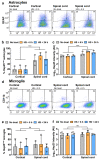
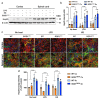
Heterogeneity of glia in different CNS regions may contribute to the selective vulnerability of neuronal populations in neurodegenerative conditions such as amyotrophic lateral sclerosis (ALS). Here, we explored regional variations in the expression of heat shock protein 25 in glia under conditions of acute and chronic stress. Hsp27 (Hsp27; murine orthologue: Hsp25) fulfils a number of cytoprotective functions and may therefore be a possible therapeutic target in ALS. We identified a subpopulation of astrocytes in primary murine mixed glial cultures that expressed Hsp25. Under basal conditions, the proportion of Hsp25-positive astrocytes was twice as high in spinal cord cultures than in cortical cultures. To explore the physiological role of the elevated Hsp25 expression in spinal cord astrocytes, we exposed cortical and spinal cord glia to acute stress, using heat stress and pro-inflammatory stimuli. Surprisingly, we observed no stress-induced increase in Hsp25 expression in either cortical or spinal cord astrocytes. Similarly, exposure to endogenous stress, as modelled in glial cultures from SOD1 G93A-ALS mice, did not increase Hsp25 expression above that observed in astrocytes from wild-type mice. In vivo, Hsp25 expression was greater under conditions of chronic stress present in the spinal cord of SOD1 G93A mice than in wild-type mice, although this increase in expression is likely to be due to the extensive gliosis that occurs in this model. Together, these results show that there are differences in the expression of Hsp25 in astrocytes in different regions of the central nervous system, but Hsp25 expression is not upregulated under acute or chronic stress conditions.
Keywords: amyotrophic lateral sclerosis; astrocytes; glia; heat shock protein 27; heat shock response; motor neuron disease.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Regional differences in the inflammatory and heat shock response in glia: implications for ALS.Cell Stress Chaperones. 2019 Sep;24(5):857-870. doi: 10.1007/s12192-019-01005-y. Epub 2019 Jun 5. Cell Stress Chaperones. 2019. PMID: 31168740 Free PMC article.
-
Histamine Is an Inducer of the Heat Shock Response in SOD1-G93A Models of ALS.Int J Mol Sci. 2019 Aug 3;20(15):3793. doi: 10.3390/ijms20153793. Int J Mol Sci. 2019. PMID: 31382568 Free PMC article.
-
Connexin 43 in astrocytes contributes to motor neuron toxicity in amyotrophic lateral sclerosis.Glia. 2016 Jul;64(7):1154-69. doi: 10.1002/glia.22989. Epub 2016 Apr 16. Glia. 2016. PMID: 27083773 Free PMC article.
-
Significance of aberrant glial cell phenotypes in pathophysiology of amyotrophic lateral sclerosis.Neurosci Lett. 2017 Jan 1;636:27-31. doi: 10.1016/j.neulet.2016.07.052. Epub 2016 Jul 26. Neurosci Lett. 2017. PMID: 27473942 Review.
-
Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology.Cell Mol Life Sci. 2009 Oct;66(20):3289-307. doi: 10.1007/s00018-009-0086-3. Epub 2009 Jul 11. Cell Mol Life Sci. 2009. PMID: 19593530 Free PMC article. Review.
Cited by
-
Perturbations of the Proteome and of Secreted Metabolites in Primary Astrocytes from the hSOD1(G93A) ALS Mouse Model.Int J Mol Sci. 2021 Jun 29;22(13):7028. doi: 10.3390/ijms22137028. Int J Mol Sci. 2021. PMID: 34209958 Free PMC article.
-
Expression of LIM domain-binding 3 (LDB3), a striated muscle Z-band alternatively spliced PDZ-motif protein in the nervous system.Sci Rep. 2023 Jan 6;13(1):270. doi: 10.1038/s41598-023-27531-5. Sci Rep. 2023. PMID: 36609526 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

