Replication of Influenza A Virus in Secondary Lymphatic Tissue Contributes to Innate Immune Activation
- PMID: 34069514
- PMCID: PMC8160763
- DOI: 10.3390/pathogens10050622
Replication of Influenza A Virus in Secondary Lymphatic Tissue Contributes to Innate Immune Activation
Abstract
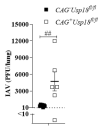
The replication of viruses in secondary lymphoid organs guarantees sufficient amounts of pattern-recognition receptor ligands and antigens to activate the innate and adaptive immune system. Viruses with broad cell tropism usually replicate in lymphoid organs; however, whether a virus with a narrow tropism relies on replication in the secondary lymphoid organs to activate the immune system remains not well studied. In this study, we used the artificial intravenous route of infection to determine whether Influenza A virus (IAV) replication can occur in secondary lymphatic organs (SLO) and whether such replication correlates with innate immune activation. Indeed, we found that IAV replicates in secondary lymphatic tissue. IAV replication was dependent on the expression of Sialic acid residues in antigen-presenting cells and on the expression of the interferon-inhibitor UBP43 (Usp18). The replication of IAV correlated with innate immune activation, resulting in IAV eradication. The genetic deletion of Usp18 curbed IAV replication and limited innate immune activation. In conclusion, we found that IAV replicates in SLO, a mechanism which allows innate immune activation.
Keywords: Influenza virus; enforced viral replication; innate immune activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
USP18 promotes innate immune responses and apoptosis in influenza A virus-infected A549 cells via cGAS-STING pathway.Virology. 2023 Aug;585:240-247. doi: 10.1016/j.virol.2023.06.012. Epub 2023 Jun 30. Virology. 2023. PMID: 37422930
-
Two separate mechanisms of enforced viral replication balance innate and adaptive immune activation.J Autoimmun. 2016 Feb;67:82-89. doi: 10.1016/j.jaut.2015.10.004. Epub 2015 Nov 7. J Autoimmun. 2016. PMID: 26553386
-
Defective Influenza A Virus RNA Products Mediate MAVS-Dependent Upregulation of Human Leukocyte Antigen Class I Proteins.J Virol. 2020 Jun 16;94(13):e00165-20. doi: 10.1128/JVI.00165-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32321802 Free PMC article.
-
Modulation of Innate Immune Responses by the Influenza A NS1 and PA-X Proteins.Viruses. 2018 Dec 12;10(12):708. doi: 10.3390/v10120708. Viruses. 2018. PMID: 30545063 Free PMC article. Review.
-
The Role of Innate Leukocytes during Influenza Virus Infection.J Immunol Res. 2019 Sep 12;2019:8028725. doi: 10.1155/2019/8028725. eCollection 2019. J Immunol Res. 2019. PMID: 31612153 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

