Synthesis and Characterization of Radiogallium-Labeled Cationic Amphiphilic Peptides as Tumor Imaging Agents
- PMID: 34069243
- PMCID: PMC8155856
- DOI: 10.3390/cancers13102388
Synthesis and Characterization of Radiogallium-Labeled Cationic Amphiphilic Peptides as Tumor Imaging Agents
Abstract
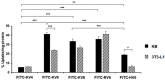
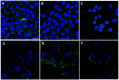
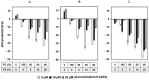
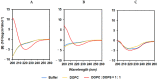
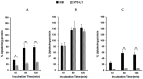
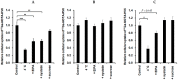
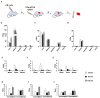
SVS-1 is a cationic amphiphilic peptide (CAP) that exhibits a preferential cytotoxicity towards cancer cells over normal cells. In this study, we developed radiogallium-labeled SVS-1 (67Ga-NOTA-KV6), as well as two SVS-1 derivatives, with the repeating KV residues replaced by RV or HV (67Ga-NOTA-RV6 and 67Ga-NOTA-HV6). All three peptides showed high accumulation in epidermoid carcinoma KB cells (53-143% uptake/mg protein). Though 67Ga-NOTA-RV6 showed the highest uptake among the three CAPs, its uptake in 3T3-L1 fibroblasts was just as high, indicating a low selectivity. In contrast, the uptake of 67Ga-NOTA-KV6 and 67Ga-NOTA-HV6 into 3T3-L1 cells was significantly lower than that in KB cells. An endocytosis inhibition study suggested that the three 67Ga-NOTA-CAPs follow distinct pathways for internalization. In the biodistribution study, the tumor uptakes were found to be 4.46%, 4.76%, and 3.18% injected dose/g of tissue (% ID/g) for 67Ga-NOTA-KV6, 67Ga-NOTA-RV6, and 67Ga-NOTA-HV6, respectively, 30 min after administration. Though the radioactivity of these peptides in tumor tissue decreased gradually, 67Ga-NOTA-KV6, 67Ga-NOTA-RV6, and 67Ga-NOTA-HV6 reached high tumor/blood ratios (7.7, 8.0, and 3.8, respectively) and tumor/muscle ratios (5.0, 3.3, and 4.0, respectively) 120 min after administration. 67Ga-NOTA-HV6 showed a lower tumor uptake than the two other tracers, but it exhibited very low levels of uptake into peripheral organs. Overall, the replacement of lysine in SVS-1 with other basic amino acids significantly influenced its binding and internalization into cancer cells, as well as its in vivo pharmacokinetic profile. The high accessibility of these peptides to tumors and their ability to target the surface membranes of cancer cells make radiolabeled CAPs excellent candidates for use in tumor theranostics.
Keywords: cancer; cationic amphiphilic peptide; molecular imaging; radiogallium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A new (68)Ga-labeled BBN peptide with a hydrophilic linker for GRPR-targeted tumor imaging.Amino Acids. 2014 Jun;46(6):1481-9. doi: 10.1007/s00726-014-1718-y. Epub 2014 Mar 17. Amino Acids. 2014. PMID: 24633452
-
The effect of macrocyclic chelators on the targeting properties of the 68Ga-labeled gastrin releasing peptide receptor antagonist PEG2-RM26.Nucl Med Biol. 2015 May;42(5):446-454. doi: 10.1016/j.nucmedbio.2014.12.009. Epub 2014 Dec 20. Nucl Med Biol. 2015. PMID: 25684649
-
Development of a New Folate-Derived Ga-68-Based PET Imaging Agent.Mol Imaging Biol. 2017 Oct;19(5):754-761. doi: 10.1007/s11307-017-1049-y. Mol Imaging Biol. 2017. PMID: 28194631 Free PMC article.
-
Synthesis and characterization of a high-affinity NOTA-conjugated bombesin antagonist for GRPR-targeted tumor imaging.Bioconjug Chem. 2013 Jul 17;24(7):1144-53. doi: 10.1021/bc300659k. Epub 2013 Jul 2. Bioconjug Chem. 2013. PMID: 23763444
-
Positron Emission Tomography Imaging of Prostate Cancer with Ga-68-Labeled Gastrin-Releasing Peptide Receptor Agonist BBN7-14 and Antagonist RM26.Bioconjug Chem. 2018 Feb 21;29(2):410-419. doi: 10.1021/acs.bioconjchem.7b00726. Epub 2018 Jan 9. Bioconjug Chem. 2018. PMID: 29254329 Free PMC article.
Cited by
-
Editorial for the Specific Issue of Radioprobes and Other Bioconjugates for Cancer Theranostics.Cancers (Basel). 2024 Jan 26;16(3):541. doi: 10.3390/cancers16030541. Cancers (Basel). 2024. PMID: 38339291 Free PMC article.
-
Conditional Antimicrobial Peptide Therapeutics.ACS Nano. 2022 Oct 25;16(10):15779-15791. doi: 10.1021/acsnano.2c04162. Epub 2022 Aug 18. ACS Nano. 2022. PMID: 35980829 Free PMC article.
References
-
- Utsugi T., Schroit A.J., Connor J., Bucana C.D., Fidler I.J. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991;51:3062–3066. - PubMed
Grants and funding
- 16H05392/Japan Society for the Promotion of Science
- 15K09895/Japan Society for the Promotion of Science
- No number/Takeda Science Foundation
- No number/Konica Minolta Imaging Science Foundation
- No Number/Mochida Memorial Foundation for Medical and Pharmaceutical Research
- No number/SGH Foundation
- No number/Terumo Foundation for Life Sciences and Arts
- No number/The Shinnihon Foundation of Advanced Medical Treatment Research
- 2017-Ippan-17/Nagasaki University and the Program of the network-type joint Usage/Research Center for Ra-diation Disaster Medical Science of Hiroshima University, Nagasaki University, and Fukushima Medical University
LinkOut - more resources
Full Text Sources
Miscellaneous

