Roles of Lytic Viral Replication and Co-Infections in the Oncogenesis and Immune Control of the Epstein-Barr Virus
- PMID: 34068598
- PMCID: PMC8126045
- DOI: 10.3390/cancers13092275
Roles of Lytic Viral Replication and Co-Infections in the Oncogenesis and Immune Control of the Epstein-Barr Virus
Abstract
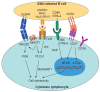
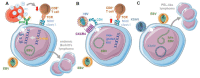
Epstein-Barr virus (EBV) is the prototypic human tumor virus whose continuous lifelong immune control is required to prevent lymphomagenesis in the more than 90% of the human adult population that are healthy carriers of the virus. Here, we review recent evidence that this immune control has not only to target latent oncogenes, but also lytic replication of EBV. Furthermore, genetic variations identify the molecular machinery of cytotoxic lymphocytes as essential for this immune control and recent studies in mice with reconstituted human immune system components (humanized mice) have begun to provide insights into the mechanistic role of these molecules during EBV infection. Finally, EBV often does not act in isolation to cause disease. Some of EBV infection-modulating co-infections, including human immunodeficiency virus (HIV) and Kaposi sarcoma-associated herpesvirus (KSHV), have been modeled in humanized mice. These preclinical in vivo models for EBV infection, lymphomagenesis, and cell-mediated immune control do not only promise a better understanding of the biology of this human tumor virus, but also the possibility to explore vaccine candidates against it.
Keywords: CD27; CD8+ T cells; Kaposi sarcoma-associated herpesvirus (KSHV); human immunodeficiency virus (HIV); humanized mice; malaria; natural killer cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Co-Infection of the Epstein-Barr Virus and the Kaposi Sarcoma-Associated Herpesvirus.Viruses. 2022 Dec 2;14(12):2709. doi: 10.3390/v14122709. Viruses. 2022. PMID: 36560713 Free PMC article. Review.
-
Persistent KSHV Infection Increases EBV-Associated Tumor Formation In Vivo via Enhanced EBV Lytic Gene Expression.Cell Host Microbe. 2017 Jul 12;22(1):61-73.e7. doi: 10.1016/j.chom.2017.06.009. Cell Host Microbe. 2017. PMID: 28704654
-
Modification of EBV Associated Lymphomagenesis and Its Immune Control by Co-Infections and Genetics in Humanized Mice.Front Immunol. 2021 Mar 23;12:640918. doi: 10.3389/fimmu.2021.640918. eCollection 2021. Front Immunol. 2021. PMID: 33833760 Free PMC article. Review.
-
The Role of Lytic Infection for Lymphomagenesis of Human γ-Herpesviruses.Front Cell Infect Microbiol. 2021 Mar 26;11:605258. doi: 10.3389/fcimb.2021.605258. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33842383 Free PMC article. Review.
-
Infection and immune control of human oncogenic γ-herpesviruses in humanized mice.Philos Trans R Soc Lond B Biol Sci. 2019 May 27;374(1773):20180296. doi: 10.1098/rstb.2018.0296. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 30955487 Free PMC article. Review.
Cited by
-
MicroRNA-focused CRISPR/Cas9 screen identifies miR-142 as a key regulator of Epstein-Barr virus reactivation.PLoS Pathog. 2024 Jun 17;20(6):e1011970. doi: 10.1371/journal.ppat.1011970. eCollection 2024 Jun. PLoS Pathog. 2024. PMID: 38885264 Free PMC article.
-
The Dual Functions of Andrographolide in the Epstein-Barr Virus-Positive Head-and-Neck Cancer Cells: The Inhibition of Lytic Reactivation of the Epstein-Barr Virus and the Induction of Cell Death.Int J Mol Sci. 2023 Nov 1;24(21):15867. doi: 10.3390/ijms242115867. Int J Mol Sci. 2023. PMID: 37958849 Free PMC article.
-
Synthetic BZLF1-targeted transcriptional activator for efficient lytic induction therapy against EBV-associated epithelial cancers.Nat Commun. 2024 May 3;15(1):3729. doi: 10.1038/s41467-024-48031-8. Nat Commun. 2024. PMID: 38702330 Free PMC article.
-
Functional Targets for Epstein-Barr Virus BART MicroRNAs in B Cell Lymphomas.Cancers (Basel). 2024 Oct 19;16(20):3537. doi: 10.3390/cancers16203537. Cancers (Basel). 2024. PMID: 39456631 Free PMC article.
-
EBV dUTPase: A Novel Modulator of Inflammation and the Tumor Microenvironment in EBV-Associated Malignancies.Cancers (Basel). 2023 Jan 30;15(3):855. doi: 10.3390/cancers15030855. Cancers (Basel). 2023. PMID: 36765813 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

