Influence of Tumor Microenvironment and Fibroblast Population Plasticity on Melanoma Growth, Therapy Resistance and Immunoescape
- PMID: 34067929
- PMCID: PMC8157224
- DOI: 10.3390/ijms22105283
Influence of Tumor Microenvironment and Fibroblast Population Plasticity on Melanoma Growth, Therapy Resistance and Immunoescape
Abstract
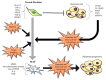
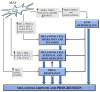
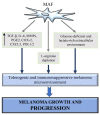
Cutaneous melanoma (CM) tissue represents a network constituted by cancer cells and tumor microenvironment (TME). A key feature of CM is the high structural and cellular plasticity of TME, allowing its evolution with disease and adaptation to cancer cell and environmental alterations. In particular, during melanoma development and progression each component of TME by interacting with each other and with cancer cells is subjected to dramatic structural and cellular modifications. These alterations affect extracellular matrix (ECM) remodelling, phenotypic profile of stromal cells, cancer growth and therapeutic response. The stromal fibroblast populations of the TME include normal fibroblasts and melanoma-associated fibroblasts (MAFs) that are highly abundant and flexible cell types interacting with melanoma and stromal cells and differently influencing CM outcomes. The shift from the normal microenvironment to TME and from normal fibroblasts to MAFs deeply sustains CM growth. Hence, in this article we review the features of the normal microenvironment and TME and describe the phenotypic plasticity of normal dermal fibroblasts and MAFs, highlighting their roles in normal skin homeostasis and TME regulation. Moreover, we discuss the influence of MAFs and their secretory profiles on TME remodelling, melanoma progression, targeted therapy resistance and immunosurveillance, highlighting the cellular interactions, the signalling pathways and molecules involved in these processes.
Keywords: fibroblasts; melanoma; melanoma-associated fibroblasts; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Insights into Melanoma Fibroblast Populations and Therapeutic Strategy Perspectives: Friends or Foes?Curr Med Chem. 2022;29(40):6159-6168. doi: 10.2174/0929867329666220620124138. Curr Med Chem. 2022. PMID: 35726413
-
The tumour microenvironment shapes dendritic cell plasticity in a human organotypic melanoma culture.Nat Commun. 2020 Jun 2;11(1):2749. doi: 10.1038/s41467-020-16583-0. Nat Commun. 2020. PMID: 32488012 Free PMC article.
-
Architecture of Cancer-Associated Fibroblasts in Tumor Microenvironment: Mapping Their Origins, Heterogeneity, and Role in Cancer Therapy Resistance.OMICS. 2020 Jun;24(6):314-339. doi: 10.1089/omi.2020.0023. OMICS. 2020. PMID: 32496970
-
Cancer associated fibroblasts: phenotypic and functional heterogeneity.Front Biosci (Landmark Ed). 2020 Mar 1;25(5):961-978. doi: 10.2741/4843. Front Biosci (Landmark Ed). 2020. PMID: 32114420 Review.
-
Stromal reprogramming: A target for tumor therapy.Life Sci. 2019 Dec 15;239:117049. doi: 10.1016/j.lfs.2019.117049. Epub 2019 Nov 12. Life Sci. 2019. PMID: 31730862 Review.
Cited by
-
Apprising Diagnostic and Prognostic Biomarkers in Cutaneous Melanoma-Persistent Updating.J Pers Med. 2022 Sep 14;12(9):1506. doi: 10.3390/jpm12091506. J Pers Med. 2022. PMID: 36143291 Free PMC article. Review.
-
Role of extracellular matrix architecture and signaling in melanoma therapeutic resistance.Front Oncol. 2022 Sep 2;12:924553. doi: 10.3389/fonc.2022.924553. eCollection 2022. Front Oncol. 2022. PMID: 36119516 Free PMC article. Review.
-
Thyroid Cancer and Fibroblasts.Cancers (Basel). 2022 Aug 29;14(17):4172. doi: 10.3390/cancers14174172. Cancers (Basel). 2022. PMID: 36077709 Free PMC article. Review.
-
Mesenchymal-Stromal Cell-like Melanoma-Associated Fibroblasts Increase IL-10 Production by Macrophages in a Cyclooxygenase/Indoleamine 2,3-Dioxygenase-Dependent Manner.Cancers (Basel). 2021 Dec 7;13(24):6173. doi: 10.3390/cancers13246173. Cancers (Basel). 2021. PMID: 34944793 Free PMC article.
-
Dermoscopic Criteria, Histopathological Correlates and Genetic Findings of Thin Melanoma on Non-Volar Skin.Genes (Basel). 2021 Aug 23;12(8):1288. doi: 10.3390/genes12081288. Genes (Basel). 2021. PMID: 34440462 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

