Annexins and Membrane Repair Dysfunctions in Muscular Dystrophies
- PMID: 34067866
- PMCID: PMC8155887
- DOI: 10.3390/ijms22105276
Annexins and Membrane Repair Dysfunctions in Muscular Dystrophies
Abstract
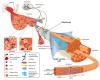
Muscular dystrophies constitute a group of genetic disorders that cause weakness and progressive loss of skeletal muscle mass. Among them, Miyoshi muscular dystrophy 1 (MMD1), limb girdle muscular dystrophy type R2 (LGMDR2/2B), and LGMDR12 (2L) are characterized by mutation in gene encoding key membrane-repair protein, which leads to severe dysfunctions in sarcolemma repair. Cell membrane disruption is a physiological event induced by mechanical stress, such as muscle contraction and stretching. Like many eukaryotic cells, muscle fibers possess a protein machinery ensuring fast resealing of damaged plasma membrane. Members of the annexins A (ANXA) family belong to this protein machinery. ANXA are small soluble proteins, twelve in number in humans, which share the property of binding to membranes exposing negatively-charged phospholipids in the presence of calcium (Ca2+). Many ANXA have been reported to participate in membrane repair of varied cell types and species, including human skeletal muscle cells in which they may play a collective role in protection and repair of the sarcolemma. Here, we discuss the participation of ANXA in membrane repair of healthy skeletal muscle cells and how dysregulation of ANXA expression may impact the clinical severity of muscular dystrophies.
Keywords: DMD; FSHD; LGMD; annexins; genetic modifiers; membrane repair; muscular dystrophy; skeletal muscle.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Defective membrane repair in dysferlin-deficient muscular dystrophy.Nature. 2003 May 8;423(6936):168-72. doi: 10.1038/nature01573. Nature. 2003. PMID: 12736685
-
Mutation finding in patients with dysferlin deficiency and role of the dysferlin interacting proteins annexin A1 and A2 in muscular dystrophies.Hum Mutat. 2005 Sep;26(3):283. doi: 10.1002/humu.9364. Hum Mutat. 2005. PMID: 16100712
-
Membrane Injury and Repair in the Muscular Dystrophies.Neuroscientist. 2015 Dec;21(6):653-68. doi: 10.1177/1073858414558336. Epub 2014 Nov 18. Neuroscientist. 2015. PMID: 25406223 Review.
-
Membrane repair of human skeletal muscle cells requires Annexin-A5.Biochim Biophys Acta. 2016 Sep;1863(9):2267-79. doi: 10.1016/j.bbamcr.2016.06.003. Epub 2016 Jun 7. Biochim Biophys Acta. 2016. PMID: 27286750
-
Dysferlin and the plasma membrane repair in muscular dystrophy.Trends Cell Biol. 2004 Apr;14(4):206-13. doi: 10.1016/j.tcb.2004.03.001. Trends Cell Biol. 2004. PMID: 15066638 Review.
Cited by
-
Inhibition of the membrane repair protein annexin-A2 prevents tumor invasion and metastasis.Cell Mol Life Sci. 2023 Dec 13;81(1):7. doi: 10.1007/s00018-023-05049-3. Cell Mol Life Sci. 2023. PMID: 38092984 Free PMC article.
-
Mechanisms of Endothelial Cell Membrane Repair: Progress and Perspectives.Cells. 2023 Nov 17;12(22):2648. doi: 10.3390/cells12222648. Cells. 2023. PMID: 37998383 Free PMC article. Review.
-
Comprehensive Proteomic Analysis of Dysferlinopathy Unveiling Molecular Mechanisms and Biomarkers Linked to Pathological Progression.CNS Neurosci Ther. 2024 Oct;30(10):e70065. doi: 10.1111/cns.70065. CNS Neurosci Ther. 2024. PMID: 39350328 Free PMC article.
-
Calpains Orchestrate Secretion of Annexin-containing Microvesicles during Membrane Repair.bioRxiv [Preprint]. 2024 Sep 6:2024.09.05.611512. doi: 10.1101/2024.09.05.611512. bioRxiv. 2024. PMID: 39282443 Free PMC article. Preprint.
-
Calcium Overload and Mitochondrial Metabolism.Biomolecules. 2022 Dec 17;12(12):1891. doi: 10.3390/biom12121891. Biomolecules. 2022. PMID: 36551319 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

