Xmrks the Spot: Fish Models for Investigating Epidermal Growth Factor Receptor Signaling in Cancer Research
- PMID: 34067095
- PMCID: PMC8150686
- DOI: 10.3390/cells10051132
Xmrks the Spot: Fish Models for Investigating Epidermal Growth Factor Receptor Signaling in Cancer Research
Abstract
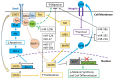
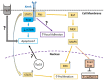
Studies conducted in several fish species, e.g., Xiphophorus hellerii (green swordtail) and Xiphophorus maculatus (southern platyfish) crosses, Oryzias latipes (medaka), and Danio rerio (zebrafish), have identified an oncogenic role for the receptor tyrosine kinase, Xmrk, a gene product closely related to the human epidermal growth factor receptor (EGFR), which is associated with a wide variety of pathological conditions, including cancer. Comparative analyses of Xmrk and EGFR signal transduction in melanoma have shown that both utilize STAT5 signaling to regulate apoptosis and cell proliferation, PI3K to modulate apoptosis, FAK to control migration, and the Ras/Raf/MEK/MAPK pathway to regulate cell survival, proliferation, and differentiation. Further, Xmrk and EGFR may also modulate similar chemokine, extracellular matrix, oxidative stress, and microRNA signaling pathways in melanoma. In hepatocellular carcinoma (HCC), Xmrk and EGFR signaling utilize STAT5 to regulate cell proliferation, and Xmrk may signal through PI3K and FasR to modulate apoptosis. At the same time, both activate the Ras/Raf/MEK/MAPK pathway to regulate cell proliferation and E-cadherin signaling. Xmrk models of melanoma have shown that inhibitors of PI3K and MEK have an anti-cancer effect, and in HCC, that the steroidal drug, adrenosterone, can prevent metastasis and recover E-cadherin expression, suggesting that fish Xmrk models can exploit similarities with EGFR signal transduction to identify and study new chemotherapeutic drugs.
Keywords: Xiphophorus; Xmrk; cancer; drug discovery; epidermal growth factor receptor; melanoma; signal transduction; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A structural model of the extracellular domain of the oncogenic EGFR variant Xmrk.Zebrafish. 2006;3(3):359-69. doi: 10.1089/zeb.2006.3.359. Zebrafish. 2006. PMID: 18377216
-
The Xmrk receptor tyrosine kinase is activated in Xiphophorus malignant melanoma.EMBO J. 1992 Nov;11(11):4239-46. doi: 10.1002/j.1460-2075.1992.tb05518.x. EMBO J. 1992. PMID: 1327761 Free PMC article.
-
Signal transduction by the oncogenic receptor tyrosine kinase Xmrk in melanoma formation of Xiphophorus.Pigment Cell Res. 1997 Feb-Apr;10(1-2):34-40. doi: 10.1111/j.1600-0749.1997.tb00463.x. Pigment Cell Res. 1997. PMID: 9170160 Review.
-
Differences in transcription and promoters of Xmrk-1 and Xmrk-2 genes suggest a role for Xmrk-2 in pigment pattern development in the platyfish, Xiphophorus maculatus.Cell Growth Differ. 1994 Jun;5(6):575-83. Cell Growth Differ. 1994. PMID: 7522032
-
Genetic, biochemical and evolutionary facets of Xmrk-induced melanoma formation in the fish Xiphophorus.Comp Biochem Physiol C Toxicol Pharmacol. 2004 Jul;138(3):281-9. doi: 10.1016/j.cca.2004.06.002. Comp Biochem Physiol C Toxicol Pharmacol. 2004. PMID: 15533786 Review.
Cited by
-
Inducible Liver Cancer Models in Transgenic Zebrafish to Investigate Cancer Biology.Cancers (Basel). 2021 Oct 14;13(20):5148. doi: 10.3390/cancers13205148. Cancers (Basel). 2021. PMID: 34680297 Free PMC article. Review.
-
Assessment of Various Standard Fish Diets on Growth and Fecundity of Platyfish (Xiphophorus maculatus) and Medaka (Oryzias latipes).Zebrafish. 2022 Oct;19(5):181-189. doi: 10.1089/zeb.2022.0004. Epub 2022 Jul 21. Zebrafish. 2022. PMID: 35862011 Free PMC article.
-
The Zebrafish model in dermatology: an update for clinicians.Discov Oncol. 2022 Jun 17;13(1):48. doi: 10.1007/s12672-022-00511-3. Discov Oncol. 2022. PMID: 35713744 Free PMC article. Review.
-
Role of Surgery in Metastatic Melanoma and Review of Melanoma Molecular Characteristics.Cells. 2024 Mar 7;13(6):465. doi: 10.3390/cells13060465. Cells. 2024. PMID: 38534309 Free PMC article. Review.
-
Effects of Dietary Koumine on Growth Performance, Intestinal Morphology, Microbiota, and Intestinal Transcriptional Responses of Cyprinus carpio.Int J Mol Sci. 2022 Oct 6;23(19):11860. doi: 10.3390/ijms231911860. Int J Mol Sci. 2022. PMID: 36233179 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

