Low RECK Expression Is Part of the Cervical Carcinogenesis Mechanisms
- PMID: 34066355
- PMCID: PMC8124470
- DOI: 10.3390/cancers13092217
Low RECK Expression Is Part of the Cervical Carcinogenesis Mechanisms
Abstract
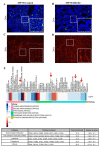
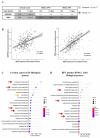
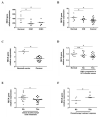
Human papillomavirus (HPV)-induced carcinogenesis comprises alterations in the expression and activity of matrix metalloproteinases (MMP) and their regulators. Reversion-inducing Cysteine-rich protein with Kazal motifs (RECK) inhibits the activation of specific metalloproteinases and its expression is frequently lost in human cancers. Here we analyzed the role of RECK in cervical carcinogenesis. Cervical cancer derived cell lines over expressing RECK were used to determine tumor kinetics as well as, cellular, immune and molecular properties in vivo. Besides, we analyzed RECK expression in cervical cancer samples. RECK over expression (RECK+) delayed tumor growth and increased overall survival in vivo. RECK+ tumors displayed an increase in lymphoid-like inflammatory infiltrating cells, reduced number and viability of tumor and endothelial cells and lower collagenase activity. RECK+ tumors exhibited an enrichment of cell adhesion processes both in the mouse model and cervical cancer clinical samples. Finally, we found that lower RECK mRNA levels were associated with cervical lesions progression and worse response to chemotherapy in cervical cancer patients. Altogether, we show that increased RECK expression reduced the tumorigenic potential of HPV-transformed cells both in vitro and in vivo, and that RECK down regulation is a consistent and clinically relevant event in the natural history of cervical cancer.
Keywords: HPV; MMP inhibitors; RECK; cervical cancer; tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
MMP-9/RECK Imbalance: A Mechanism Associated with High-Grade Cervical Lesions and Genital Infection by Human Papillomavirus and Chlamydia trachomatis.Cancer Epidemiol Biomarkers Prev. 2015 Oct;24(10):1539-47. doi: 10.1158/1055-9965.EPI-15-0420. Epub 2015 Aug 10. Cancer Epidemiol Biomarkers Prev. 2015. PMID: 26261088
-
Downregulation of reversion-inducing cysteine-rich protein with Kazal motifs in malignant melanoma: inverse correlation with membrane-type 1-matrix metalloproteinase and tissue inhibitor of metalloproteinase 2.Melanoma Res. 2014 Feb;24(1):32-9. doi: 10.1097/CMR.0000000000000039. Melanoma Res. 2014. PMID: 24335752
-
microRNA-200b and microRNA-200c promote colorectal cancer cell proliferation via targeting the reversion-inducing cysteine-rich protein with Kazal motifs.RNA Biol. 2015;12(3):276-89. doi: 10.1080/15476286.2015.1017208. RNA Biol. 2015. PMID: 25826661 Free PMC article.
-
RECKing MMP: relevance of reversion-inducing cysteine-rich protein with kazal motifs as a prognostic marker and therapeutic target for cancer (a review).Anticancer Agents Med Chem. 2012 Sep;12(7):718-25. doi: 10.2174/187152012802650237. Anticancer Agents Med Chem. 2012. PMID: 22292753 Review.
-
The RECK gene and biological malignancy--its significance in angiogenesis and inhibition of matrix metalloproteinases.Anticancer Res. 2014 Aug;34(8):3867-73. Anticancer Res. 2014. PMID: 25075007 Review.
Cited by
-
Identification of RECK as a protective prognostic indicator and a tumor suppressor through regulation of the ERK/MAPK signaling pathway in gastric cancer.J Transl Med. 2023 Oct 30;21(1):766. doi: 10.1186/s12967-023-04644-z. J Transl Med. 2023. PMID: 37904179 Free PMC article.
-
The regulation roles of miRNAs in Helicobacter pylori infection.Clin Transl Oncol. 2023 Jul;25(7):1929-1939. doi: 10.1007/s12094-023-03094-9. Epub 2023 Feb 13. Clin Transl Oncol. 2023. PMID: 36781601 Review.
-
RECK/GPR124-driven WNT signaling in pancreatic and gastric cancer cells.Cancer Sci. 2024 Sep;115(9):3013-3025. doi: 10.1111/cas.16258. Epub 2024 Jun 26. Cancer Sci. 2024. PMID: 38923741 Free PMC article.
-
MiR-21 Regulates Growth and Migration of Cervical Cancer Cells by RECK Signaling Pathway.Int J Mol Sci. 2024 Apr 6;25(7):4086. doi: 10.3390/ijms25074086. Int J Mol Sci. 2024. PMID: 38612895 Free PMC article.
-
Pin1/YAP pathway mediates matrix stiffness-induced epithelial-mesenchymal transition driving cervical cancer metastasis via a non-Hippo mechanism.Bioeng Transl Med. 2022 Jul 7;8(1):e10375. doi: 10.1002/btm2.10375. eCollection 2023 Jan. Bioeng Transl Med. 2022. PMID: 36684109 Free PMC article.
References
-
- Schlecht N.F., Kulaga S., Robitaille J., Ferreira S., Santos M., Miyamura R.A., Duarte-Franco E., Rohan T.E., Ferenczy A., Villa L.L., et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA. 2001;286:3106–3114. doi: 10.1001/jama.286.24.3106. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

