Long-Term Preservation and Storage of Faecal Samples in Whatman® Cards for PCR Detection and Genotyping of Giardia duodenalis and Cryptosporidium hominis
- PMID: 34065892
- PMCID: PMC8151430
- DOI: 10.3390/ani11051369
Long-Term Preservation and Storage of Faecal Samples in Whatman® Cards for PCR Detection and Genotyping of Giardia duodenalis and Cryptosporidium hominis
Abstract
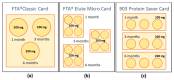
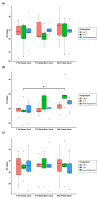
Preservation and conservation of biological specimens, including faecal samples, is a challenge in remote areas or poor-resource settings where the cold chain cannot be maintained. This study aims at evaluating the suitability of filter cards for long-term storage of faecal samples of animal and human origin positive to the diarrhoea-causing protozoan parasites, Giardia duodenalis and Cryptosporidium hominis. Three commercially available Whatman® Filter Cards were comparatively assessed: the FTA® Classic Card, the FTA® Elute Micro Card, and the 903 Protein Saver Card. Human faecal samples positive to G. duodenalis (n = 5) and C. hominis (n = 5) were used to impregnate the selected cards at given storage (1 month, 3 months, and 6 months) periods and temperature (-20 °C, 4 °C, and room temperature) conditions. Parasite DNA was detected by PCR-based methods. Sensitivity assays and quality control procedures to assess suitability for genotyping purposes were conducted. Overall, all three Whatman® cards were proven useful for the detection and molecular characterisation of G. duodenalis and C. hominis under the evaluated conditions. Whatman® cards represent a simple, safe, and cost-effective option for the transportation, preservation, and storage of faecal samples without the need of the cold chain.
Keywords: Cryptosporidium hominis; Giardia duodenalis; PCR; faeces; filter card; preservation; storage; transportation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Whatman Protein Saver Cards for Storage and Detection of Parasitic Enteropathogens.Am J Trop Med Hyg. 2018 Dec;99(6):1613-1618. doi: 10.4269/ajtmh.18-0538. Am J Trop Med Hyg. 2018. PMID: 30398140 Free PMC article.
-
Performance of three rapid diagnostic tests for the detection of Cryptosporidium spp. and Giardia duodenalis in children with severe acute malnutrition and diarrhoea.Infect Dis Poverty. 2019 Nov 28;8(1):96. doi: 10.1186/s40249-019-0609-6. Infect Dis Poverty. 2019. PMID: 31775877 Free PMC article.
-
Usage of FTA® Classic Cards for Safe Storage, Shipment, and Detection of Arboviruses.Microorganisms. 2022 Jul 18;10(7):1445. doi: 10.3390/microorganisms10071445. Microorganisms. 2022. PMID: 35889164 Free PMC article.
-
Comparative performance evaluation of four commercial multiplex real-time PCR assays for the detection of the diarrhoea-causing protozoa Cryptosporidium hominis/parvum, Giardia duodenalis and Entamoeba histolytica.PLoS One. 2019 Apr 8;14(4):e0215068. doi: 10.1371/journal.pone.0215068. eCollection 2019. PLoS One. 2019. PMID: 30958837 Free PMC article.
-
Is real-time PCR-based diagnosis similar in performance to routine parasitological examination for the identification of Giardia intestinalis, Cryptosporidium parvum/Cryptosporidium hominis and Entamoeba histolytica from stool samples? Evaluation of a new commercial multiplex PCR assay and literature review.Clin Microbiol Infect. 2016 Feb;22(2):190.e1-190.e8. doi: 10.1016/j.cmi.2015.10.019. Epub 2015 Nov 6. Clin Microbiol Infect. 2016. PMID: 26548509 Review.
Cited by
-
Zoonoses and Wildlife: One Health Approach.Animals (Basel). 2022 Feb 15;12(4):480. doi: 10.3390/ani12040480. Animals (Basel). 2022. PMID: 35203188 Free PMC article.
References
-
- Paulos S., Mateo M., de Lucio A., Hernández-de Mingo M., Bailo B., Saugar J.M., Cardona G.A., Fuentes I., Mateo M., Carmena D. Evaluation of five commercial methods for the extraction and purification of DNA from human faecal samples for downstream molecular detection of the enteric protozoan parasites Cryptosporidium spp., Giardia duodenalis, and Entamoeba spp. J. Microbiol. Methods. 2016;127:68–73. doi: 10.1016/j.mimet.2016.05.020. - DOI - PubMed
-
- Guthrie R., Susi A. A simple phenylalanine method for detecting phenylketonuria in large population of newborn infants. Pediatrics. 1963;32:338–343. - PubMed
-
- GE Healthcare Life Sciences Your Forensic Samples, Our Experience. [(accessed on 1 May 2021)]; Available online: www.gelifesciences.com/forensics.
LinkOut - more resources
Full Text Sources

