The Emerging Role of Neutrophils in the Pathogenesis of Thrombosis in COVID-19
- PMID: 34065210
- PMCID: PMC8161034
- DOI: 10.3390/ijms22105368
The Emerging Role of Neutrophils in the Pathogenesis of Thrombosis in COVID-19
Abstract
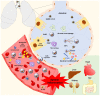
Previous studies have shown that COVID-19 leads to thrombotic complications, which have been associated with high morbidity and mortality rates. Neutrophils are the largest population of white blood cells and play a pivotal role in innate immunity. During an infection, neutrophils migrate from circulation to the infection site, contributing to killing pathogens. This mechanism is regulated by chemokines such as IL-8. Moreover, it was shown that neutrophils play an important role in thromboinflammation. Through a diverse repertoire of mechanisms, neutrophils, apart from directly killing pathogens, are able to activate the formation of thrombi. In COVID-19 patients, neutrophil activation promotes neutrophil extracellular trap (NET) formation, platelet aggregation, and cell damage. Furthermore, neutrophils participate in the pathogenesis of endothelitis. Overall, this review summarizes recent progress in research on the pathogenesis of COVID-19, highlighting the role of the prothrombotic action of neutrophils in NET formation.
Keywords: COVID-19; NETs; SARS CoV-2; immunothrombosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Neutrophil Extracellular Traps (NETs) in Severe SARS-CoV-2 Lung Disease.Int J Mol Sci. 2021 Aug 17;22(16):8854. doi: 10.3390/ijms22168854. Int J Mol Sci. 2021. PMID: 34445556 Free PMC article. Review.
-
COVID-19 and Neutrophils: The Relationship between Hyperinflammation and Neutrophil Extracellular Traps.Mediators Inflamm. 2020 Dec 2;2020:8829674. doi: 10.1155/2020/8829674. eCollection 2020. Mediators Inflamm. 2020. PMID: 33343232 Free PMC article. Review.
-
NETosis and Neutrophil Extracellular Traps in COVID-19: Immunothrombosis and Beyond.Front Immunol. 2022 Mar 2;13:838011. doi: 10.3389/fimmu.2022.838011. eCollection 2022. Front Immunol. 2022. PMID: 35309344 Free PMC article. Review.
-
Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome.Blood. 2020 Sep 3;136(10):1169-1179. doi: 10.1182/blood.2020007008. Blood. 2020. PMID: 32597954 Free PMC article.
-
Neutrophil Extracellular Traps (NETs) and Covid-19: A new frontiers for therapeutic modality.Int Immunopharmacol. 2022 Mar;104:108516. doi: 10.1016/j.intimp.2021.108516. Epub 2022 Jan 6. Int Immunopharmacol. 2022. PMID: 35032828 Free PMC article. Review.
Cited by
-
Biomarkers of cell damage, neutrophil and macrophage activation associated with in-hospital mortality in geriatric COVID-19 patients.Immun Ageing. 2022 Dec 15;19(1):65. doi: 10.1186/s12979-022-00315-7. Immun Ageing. 2022. PMID: 36522763 Free PMC article.
-
The central role of neutrophil extracellular traps (NETs) and by-products in COVID-19 related pulmonary thrombosis.Immun Inflamm Dis. 2023 Aug;11(8):e949. doi: 10.1002/iid3.949. Immun Inflamm Dis. 2023. PMID: 37647446 Free PMC article. Review.
-
Pathogenic Basis of Thromboinflammation and Endothelial Injury in COVID-19: Current Findings and Therapeutic Implications.Int J Mol Sci. 2021 Nov 8;22(21):12081. doi: 10.3390/ijms222112081. Int J Mol Sci. 2021. PMID: 34769508 Free PMC article. Review.
-
Neutrophil activation and neutrophil extracellular traps (NETs) in COVID-19 ARDS and immunothrombosis.Eur J Immunol. 2023 Jan;53(1):e2250010. doi: 10.1002/eji.202250010. Epub 2022 Nov 1. Eur J Immunol. 2023. PMID: 36239164 Free PMC article. Review.
-
Phenotypic alteration of low-density granulocytes in people with pulmonary post-acute sequalae of SARS-CoV-2 infection.Front Immunol. 2022 Dec 15;13:1076724. doi: 10.3389/fimmu.2022.1076724. eCollection 2022. Front Immunol. 2022. PMID: 36591237 Free PMC article.
References
-
- WHO Coronavirus (COVID-19) Dashboard. [(accessed on 28 March 2021)]; Available online: https://covid19.who.int.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

