Triple-Knockout, Synuclein-Free Mice Display Compromised Lipid Pattern
- PMID: 34064018
- PMCID: PMC8196748
- DOI: 10.3390/molecules26113078
Triple-Knockout, Synuclein-Free Mice Display Compromised Lipid Pattern
Abstract
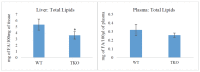
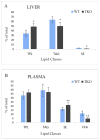
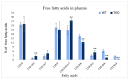
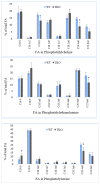
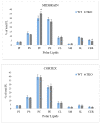
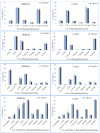
Recent studies have implicated synucleins in several reactions during the biosynthesis of lipids and fatty acids in addition to their recognised role in membrane lipid binding and synaptic functions. These are among aspects of decreased synuclein functions that are still poorly acknowledged especially in regard to pathogenesis in Parkinson's disease. Here, we aimed to add to existing knowledge of synuclein deficiency (i.e., the lack of all three family members), with respect to changes in fatty acids and lipids in plasma, liver, and two brain regions in triple synuclein-knockout (TKO) mice. We describe changes of long-chain polyunsaturated fatty acids (LCPUFA) and palmitic acid in liver and plasma, reduced triacylglycerol (TAG) accumulation in liver and non-esterified fatty acids in plasma of synuclein free mice. In midbrain, we observed counterbalanced changes in the relative concentrations of phosphatidylcholine (PC) and cerebrosides (CER). We also recorded a notable reduction in ethanolamine plasmalogens in the midbrain of synuclein free mice, which is an important finding since the abnormal ether lipid metabolism usually associated with neurological disorders. In summary, our data demonstrates that synuclein deficiency results in alterations of the PUFA synthesis, storage lipid accumulation in the liver, and the reduction of plasmalogens and CER, those polar lipids which are principal compounds of lipid rafts in many tissues. An ablation of all three synuclein family members causes more profound changes in lipid metabolism than changes previously shown to be associated with γ-synuclein deficiency alone. Possible mechanisms by which synuclein deficiency may govern the reported modifications of lipid metabolism in TKO mice are proposed and discussed.
Keywords: fatty acids; non-polar lipids; phospholipids; synucleins; triple-knockout mouse model.
Conflict of interest statement
The authors declared none to disclose regarding conflict of interest.
Figures


Similar articles
-
Fatty acid incorporation is decreased in astrocytes cultured from alpha-synuclein gene-ablated mice.J Neurochem. 2005 Aug;94(3):839-49. doi: 10.1111/j.1471-4159.2005.03247.x. J Neurochem. 2005. PMID: 16033426
-
Alpha-synuclein gene deletion decreases brain palmitate uptake and alters the palmitate metabolism in the absence of alpha-synuclein palmitate binding.Biochemistry. 2005 Jun 14;44(23):8251-9. doi: 10.1021/bi0502137. Biochemistry. 2005. PMID: 15938614
-
αβγ-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction.Proc Natl Acad Sci U S A. 2010 Nov 9;107(45):19573-8. doi: 10.1073/pnas.1005005107. Epub 2010 Oct 25. Proc Natl Acad Sci U S A. 2010. PMID: 20974939 Free PMC article.
-
Molecular and cellular biology of synucleins.Int Rev Cell Mol Biol. 2008;270:225-317. doi: 10.1016/S1937-6448(08)01406-8. Int Rev Cell Mol Biol. 2008. PMID: 19081538 Review.
-
Synucleins: are they two-edged swords?J Neurosci Res. 2013 Feb;91(2):161-6. doi: 10.1002/jnr.23149. Epub 2012 Nov 14. J Neurosci Res. 2013. PMID: 23150342 Review.
Cited by
-
Beta2-adrenoreceptor agonist clenbuterol produces transient decreases in alpha-synuclein mRNA but no long-term reduction in protein.NPJ Parkinsons Dis. 2022 May 24;8(1):61. doi: 10.1038/s41531-022-00322-x. NPJ Parkinsons Dis. 2022. PMID: 35610264 Free PMC article.
References
-
- Connor-Robson N., Peters O., Millership S., Ninkina N., Buchman V.L. Combinational losses of synucleins reveal their differential requirements for compensating age-dependent alterations in motor behaviour and dopamine metabolism. Neurobiol. Aging. 2016;46:107–112. doi: 10.1016/j.neurobiolaging.2016.06.020. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

