Antidepressants Differentially Regulate Intracellular Signaling from α1-Adrenergic Receptor Subtypes In Vitro
- PMID: 34062902
- PMCID: PMC8124549
- DOI: 10.3390/ijms22094817
Antidepressants Differentially Regulate Intracellular Signaling from α1-Adrenergic Receptor Subtypes In Vitro
Abstract
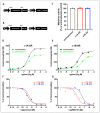
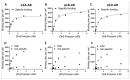
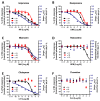
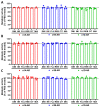
Currently utilized antidepressants have limited effectiveness and frequently incur undesired effects. Most antidepressants are thought to act via the inhibition of monoamine reuptake; however, direct binding to monoaminergic receptors has been proposed to contribute to both their clinical effectiveness and their side effects, or lack thereof. Among the target receptors of antidepressants, α1‑adrenergic receptors (ARs) have been implicated in depression etiology, antidepressant action, and side effects. However, differences in the direct effects of antidepressants on signaling from the three subtypes of α1-ARs, namely, α1A-, α1B- and α1D‑ARs, have been little explored. We utilized cell lines overexpressing α1A-, α1B- or α1D-ARs to investigate the effects of the antidepressants imipramine (IMI), desipramine (DMI), mianserin (MIA), reboxetine (REB), citalopram (CIT) and fluoxetine (FLU) on noradrenaline-induced second messenger generation by those receptors. We found similar orders of inhibition at α1A-AR (IMI < DMI < CIT < MIA < REB) and α1D‑AR (IMI = DMI < CIT < MIA), while the α1B-AR subtype was the least engaged subtype and was inhibited with low potency by three drugs (MIA < IMI = DMI). In contrast to their direct antagonistic effects, prolonged incubation with IMI and DMI increased the maximal response of the α1B-AR subtype, and the CIT of both the α1A- and the α1B-ARs. Our data demonstrate a complex, subtype-specific modulation of α1-ARs by antidepressants of different groups.
Keywords: G-protein-coupled receptor; alpha1-adrenergic receptor subtypes; antagonist; antidepressants; citalopram; desipramine; imipramine; inositol phosphate; mianserin; second messenger.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Differential activation of mitogen-activated protein kinase pathways in PC12 cells by closely related alpha1-adrenergic receptor subtypes.J Neurochem. 1999 Jun;72(6):2388-96. doi: 10.1046/j.1471-4159.1999.0722388.x. J Neurochem. 1999. PMID: 10349848
-
Chronic treatment with citalopram does not affect the expression of alpha1-adrenergic receptor (alpha1-AR) subtypes.Pol J Pharmacol. 2004 Nov-Dec;56(6):831-6. Pol J Pharmacol. 2004. PMID: 15662097
-
Dominance of the alpha1B-adrenergic receptor and its subcellular localization in human and TRAMP prostate cancer cell lines.J Recept Signal Transduct Res. 2007;27(1):27-45. doi: 10.1080/10799890601087487. J Recept Signal Transduct Res. 2007. PMID: 17365508
-
[Antidepressant drugs and central monoaminergic receptors].Yakubutsu Seishin Kodo. 1985 Dec;5(4):303-19. Yakubutsu Seishin Kodo. 1985. PMID: 2870595 Review. Japanese.
-
Temporal sequence of changes in central noradrenergic system of rat after prolonged antidepressant treatment: receptor desensitization and neurotransmitter interactions.Neuropharmacology. 1983 Mar;22(3 Spec No):415-24. doi: 10.1016/0028-3908(83)90191-0. Neuropharmacology. 1983. PMID: 6304559 Review.
References
-
- World Health Organization . Depression and Other Common Mental Disorders: Global Health Estimates. World Health Organization; Geneva, Switzerland: 2017.
-
- Wittchen H.U., Jacobi F., Rehm J., Gustavsson A., Svensson M., Jönsson B., Olesen J., Allgulander C., Alonso J., Faravelli C., et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011;21:655–679. doi: 10.1016/j.euroneuro.2011.07.018. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

