TLR7 Ligation Inhibits TLR8 Responsiveness in IL-27-Primed Human THP-1 Monocytes and Macrophages
- PMID: 34058746
- PMCID: PMC8613552
- DOI: 10.1159/000515738
TLR7 Ligation Inhibits TLR8 Responsiveness in IL-27-Primed Human THP-1 Monocytes and Macrophages
Abstract
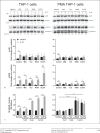
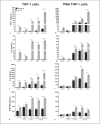
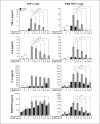
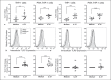
Regulation of proinflammatory cytokine expression is critical in the face of single-stranded RNA (ssRNA) virus infections. Many viruses, including coronavirus and influenza virus, wreak havoc on the control of cytokine expression, leading to the formation of detrimental cytokine storms. Understanding the regulation and interplay between inflammatory cytokines is critical to the identification of targets involved in controlling the induction of cytokine expression. In this study, we focused on how the antiviral cytokine interleukin-27 (IL-27) regulates signal transduction downstream of Toll-like receptor 7 (TLR7) and TLR8 ligation, which recognize endosomal single-stranded RNA. Given that IL-27 alters bacterial-sensing TLR expression on myeloid cells and can inhibit replication of single-stranded RNA viruses, we investigated whether IL-27 affects expression and function of TLR7 and TLR8. Analysis of IL-27-treated THP-1 monocytic cells and THP-1-derived macrophages revealed changes in mRNA and protein expression of TLR7 and TLR8. Although treatment with IL-27 enhanced TLR7 expression, only TLR8-mediated cytokine secretion was amplified. Furthermore, we demonstrated that imiquimod, a TLR7 agonist, inhibited cytokine and chemokine production induced by a TLR8 agonist, TL8-506. Delineating the immunomodulatory role of IL-27 on TLR7 and TLR8 responses provides insight into how myeloid cell TLR-mediated responses are regulated during virus infection.
Keywords: Inflammation; Interferon; Interleukin-27; Macrophages; Monocytes; Toll-like receptor; Toll-like receptor 7; Toll-like receptor 8.
© 2021 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
TLR7 and TLR8 activate distinct pathways in monocytes during RNA virus infection.Sci Signal. 2019 Oct 29;12(605):eaaw1347. doi: 10.1126/scisignal.aaw1347. Sci Signal. 2019. PMID: 31662487
-
Differences in TLR7/8 activation between monocytes and macrophages.Biochem Biophys Res Commun. 2018 Feb 26;497(1):319-325. doi: 10.1016/j.bbrc.2018.02.079. Epub 2018 Feb 12. Biochem Biophys Res Commun. 2018. PMID: 29448098
-
TLR7 and TLR8 ligands and antiphospholipid antibodies show synergistic effects on the induction of IL-1beta and caspase-1 in monocytes and dendritic cells.Immunobiology. 2009;214(8):683-91. doi: 10.1016/j.imbio.2008.12.003. Epub 2009 Feb 26. Immunobiology. 2009. PMID: 19249118
-
Antiviral signaling through pattern recognition receptors.J Biochem. 2007 Feb;141(2):137-45. doi: 10.1093/jb/mvm032. Epub 2006 Dec 26. J Biochem. 2007. PMID: 17190786 Review.
-
RNA recognition via TLR7 and TLR8.Handb Exp Pharmacol. 2008;(183):71-86. doi: 10.1007/978-3-540-72167-3_4. Handb Exp Pharmacol. 2008. PMID: 18071655 Review.
Cited by
-
The Relevance of TLR8 in Viral Infections.Pathogens. 2022 Jan 22;11(2):134. doi: 10.3390/pathogens11020134. Pathogens. 2022. PMID: 35215078 Free PMC article. Review.
-
IL-27 Induces CCL5 Production by T Lymphocytes, Which Contributes to Antitumor Activity.J Immunol. 2022 May 1;208(9):2239-2245. doi: 10.4049/jimmunol.2100885. Epub 2022 Apr 13. J Immunol. 2022. PMID: 35418466 Free PMC article.
-
Some Like It Hot.J Innate Immun. 2021;13(6):321-322. doi: 10.1159/000520270. Epub 2021 Oct 26. J Innate Immun. 2021. PMID: 34724673 Free PMC article. No abstract available.
-
Antiviral Activities of Interleukin-27: A Partner for Interferons?Front Immunol. 2022 May 10;13:902853. doi: 10.3389/fimmu.2022.902853. eCollection 2022. Front Immunol. 2022. PMID: 35634328 Free PMC article. Review.
-
Distinct SARS-CoV-2 RNA fragments activate Toll-like receptors 7 and 8 and induce cytokine release from human macrophages and microglia.Front Immunol. 2023 Jan 13;13:1066456. doi: 10.3389/fimmu.2022.1066456. eCollection 2022. Front Immunol. 2023. PMID: 36713399 Free PMC article.
References
-
- Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32((3)):305–15. - PubMed
-
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303((5663)):1529–31. - PubMed
-
- Qian C, Cao X. Regulation of Toll-like receptor signaling pathways in innate immune responses. Ann N Y Acad Sci. 2013;1283:67–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

