Genetic toxicity testing using human in vitro organotypic airway cultures: Assessing DNA damage with the CometChip and mutagenesis by Duplex Sequencing
- PMID: 34050964
- PMCID: PMC8251634
- DOI: 10.1002/em.22444
Genetic toxicity testing using human in vitro organotypic airway cultures: Assessing DNA damage with the CometChip and mutagenesis by Duplex Sequencing
Abstract
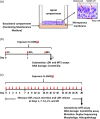
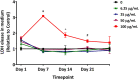
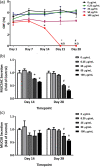
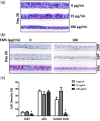
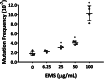
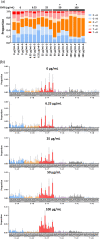
The organotypic human air-liquid-interface (ALI) airway tissue model has been used as an in vitro cell culture system for evaluating the toxicity of inhaled substances. ALI airway cultures are highly differentiated, which has made it challenging to evaluate genetic toxicology endpoints. In the current study, we assayed DNA damage with the high-throughput CometChip assay and quantified mutagenesis with Duplex Sequencing, an error-corrected next-generation sequencing method capable of detecting a single mutation per 107 base pairs. Fully differentiated human ALI airway cultures were treated from the basolateral side with 6.25 to 100 μg/mL ethyl methanesulfonate (EMS) over a period of 28 days. CometChip assays were conducted after 3 and 28 days of treatment, and Duplex Sequencing after 28 days of treatment. Treating the airway cultures with EMS resulted in time- and concentration-dependent increases in DNA damage and a concentration-dependent increase in mutant frequency. The mutations observed in the EMS-treated cultures were predominantly C → T transitions and exhibited a unique trinucleotide signature relative to the negative control. Measurement of physiological endpoints indicated that the EMS treatments had no effect on anti-p63-positive basal cell frequency, but produced concentration-responsive increases in cytotoxicity and perturbations in cell morphology, along with concentration-responsive decreases in culture viability, goblet cell and anti-Ki67-positive proliferating cell frequency, cilia beating frequency, and mucin secretion. The results indicate that a unified 28-day study can be used to measure several important safety endpoints in physiologically relevant human in vitro ALI airway cultures, including DNA damage, mutagenicity, and tissue-specific general toxicity.
Keywords: DNA damage; error-corrected next generation sequencing (ecNGS); ethyl methanesulfonate (EMS); human in vitro air-liquid-interface (ALI) airway epithelial tissue model; mutagenesis.
© 2021 The Authors. Emergency Medicine Australasia published by John Wiley & Sons Australia, Ltd on behalf of Australasian College for Emergency Medicine. This article has been contributed to by US Government employees and their work is in the public domain in the USA.
Conflict of interest statement
E.K.S., T.H.S., Z.K.N., C.C.V., J.F., L.N.W., and J.J.S. are employees and equity holders at TwinStrand Biosciences, Inc. E.K.S., C.C.V., L.N.W., and J.J.S. are authors on one or more Duplex Sequencing‐related patents.
Figures

Similar articles
-
Repeat treatment of organotypic airway cultures with ethyl methanesulfonate causes accumulation of somatic cell mutations without expansion of bronchial-carcinoma-specific cancer driver mutations.Mutat Res Genet Toxicol Environ Mutagen. 2024 Jul;897:503786. doi: 10.1016/j.mrgentox.2024.503786. Epub 2024 Jun 24. Mutat Res Genet Toxicol Environ Mutagen. 2024. PMID: 39054009
-
Detection of opiate-enhanced increases in DNA damage, HPRT mutants, and the mutation frequency in human HUT-78 cells.Environ Mol Mutagen. 1994;23(1):37-44. doi: 10.1002/em.2850230107. Environ Mol Mutagen. 1994. PMID: 8125082
-
Ethyl methanesulfonate toxicity in Viracept--a comprehensive human risk assessment based on threshold data for genotoxicity.Toxicol Lett. 2009 Nov 12;190(3):317-29. doi: 10.1016/j.toxlet.2009.04.003. Epub 2009 Apr 10. Toxicol Lett. 2009. PMID: 19443141
-
Error-corrected next generation sequencing - Promises and challenges for genotoxicity and cancer risk assessment.Mutat Res Rev Mutat Res. 2023 Jul-Dec;792:108466. doi: 10.1016/j.mrrev.2023.108466. Epub 2023 Aug 27. Mutat Res Rev Mutat Res. 2023. PMID: 37643677 Review.
-
Invited review: human air-liquid-interface organotypic airway tissue models derived from primary tracheobronchial epithelial cells-overview and perspectives.In Vitro Cell Dev Biol Anim. 2021 Feb;57(2):104-132. doi: 10.1007/s11626-020-00517-7. Epub 2020 Nov 11. In Vitro Cell Dev Biol Anim. 2021. PMID: 33175307 Free PMC article. Review.
Cited by
-
Adopting duplex sequencing technology for genetic toxicity testing: A proof-of-concept mutagenesis experiment with N-ethyl-N-nitrosourea (ENU)-exposed rats.Mutat Res Genet Toxicol Environ Mutagen. 2023 Oct;891:503669. doi: 10.1016/j.mrgentox.2023.503669. Epub 2023 Aug 3. Mutat Res Genet Toxicol Environ Mutagen. 2023. PMID: 37770135 Free PMC article.
-
Adopting Duplex Sequencing™ Technology for Genetic Toxicity Testing: A Proof-of-Concept Mutagenesis Experiment with N-Ethyl-N-Nitrosourea (ENU)-Exposed Rats.bioRxiv [Preprint]. 2023 May 9:2023.05.08.539833. doi: 10.1101/2023.05.08.539833. bioRxiv. 2023. Update in: Mutat Res Genet Toxicol Environ Mutagen. 2023 Oct;891:503669. doi: 10.1016/j.mrgentox.2023.503669 PMID: 37214853 Free PMC article. Updated. Preprint.
-
Frequencies and spectra of aflatoxin B1-induced mutations in liver genomes of NEIL1-deficient mice as revealed by duplex sequencing.NAR Mol Med. 2024 May 17;1(2):ugae006. doi: 10.1093/narmme/ugae006. eCollection 2024 Apr. NAR Mol Med. 2024. PMID: 38779538 Free PMC article.
-
Frequency and spectrum of mutations in human sperm measured using duplex sequencing correlate with trio-based de novo mutation analyses.Sci Rep. 2024 Oct 8;14(1):23134. doi: 10.1038/s41598-024-73587-2. Sci Rep. 2024. PMID: 39379474 Free PMC article.
-
Evaluating the mutagenicity of N-nitrosodimethylamine in 2D and 3D HepaRG cell cultures using error-corrected next generation sequencing.Arch Toxicol. 2024 Jun;98(6):1919-1935. doi: 10.1007/s00204-024-03731-4. Epub 2024 Apr 7. Arch Toxicol. 2024. PMID: 38584193 Free PMC article.
References
-
- BéruBé, K. , Aufderheide, M. , Breheny, D. , Clothier, R. , Combes, R. , Duffin, R. et al. (2009) In vitro models of inhalation toxicity and disease: the report of a FRAME workshop. ATLA, 37, 89–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

