Sensory Neurons, Neuroimmunity, and Pain Modulation by Sex Hormones
- PMID: 34049389
- PMCID: PMC8237991
- DOI: 10.1210/endocr/bqab109
Sensory Neurons, Neuroimmunity, and Pain Modulation by Sex Hormones
Abstract
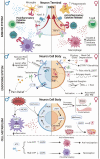
The inclusion of women in preclinical pain studies has become more commonplace in the last decade as the National Institutes of Health (NIH) released its "Sex as a Biological Variable" mandate. Presumably, basic researchers have not had a comprehensive understanding about neuroimmune interactions in half of the population and how hormones play a role in this. To date, we have learned that sex hormones contribute to sexual differentiation of the nervous system and sex differences in behavior throughout the lifespan; however, the cycling of sex hormones does not always explain these differences. Here, we highlight recent advances in our understanding of sex differences and how hormones and immune interactions influence sensory neuron activity to contribute to physiology and pain. Neuroimmune mechanisms may be mediated by different cell types in each sex, as the actions of immune cells are sexually dimorphic. Unfortunately, the majority of studies assessing neuronal contributions to immune function have been limited to males, so it is unclear if the mechanisms are similar in females. Finally, pathways that control cellular metabolism, like nuclear receptors, have been shown to play a regulatory role both in pain and inflammation. Overall, communication between the neuroimmune and endocrine systems modulate pain signaling in a sex-dependent manner, but more research is needed to reveal nuances of these mechanisms.
Keywords: bidirectional; hormone; neuroendocrine; neuroimmune; pain; sensory neuron; sex differences.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
The influence of sex on neuroimmune communication, pain, and physiology.Biol Sex Differ. 2024 Oct 22;15(1):82. doi: 10.1186/s13293-024-00660-w. Biol Sex Differ. 2024. PMID: 39439003 Free PMC article. Review.
-
Sex differences in neuro(auto)immunity and chronic sciatic nerve pain.Biol Sex Differ. 2020 Nov 12;11(1):62. doi: 10.1186/s13293-020-00339-y. Biol Sex Differ. 2020. PMID: 33183347 Free PMC article. Review.
-
Neuroendocrine-Immune Crosstalk Shapes Sex-Specific Brain Development.Endocrinology. 2020 Jun 1;161(6):bqaa055. doi: 10.1210/endocr/bqaa055. Endocrinology. 2020. PMID: 32270188 Free PMC article. Review.
-
Sex differences in neuroimmunity and pain.J Neurosci Res. 2017 Jan 2;95(1-2):500-508. doi: 10.1002/jnr.23831. J Neurosci Res. 2017. PMID: 27870397 Review.
-
Neuroendocrine-immune (NEI) circuitry from neuron-glial interactions to function: Focus on gender and HPA-HPG interactions on early programming of the NEI system.Immunol Cell Biol. 2001 Aug;79(4):400-17. doi: 10.1046/j.1440-1711.2001.01030.x. Immunol Cell Biol. 2001. PMID: 11488988 Review.
Cited by
-
IRG1/itaconate increases IL-10 release to alleviate mechanical and thermal hypersensitivity in mice after nerve injury.Front Immunol. 2022 Oct 13;13:1012442. doi: 10.3389/fimmu.2022.1012442. eCollection 2022. Front Immunol. 2022. PMID: 36311727 Free PMC article.
-
Battle of the Blocks: Which Pain Management Technique Triumphs in Gender-Affirming Bilateral Mastectomies?J Clin Med Res. 2024 Jun;16(6):284-292. doi: 10.14740/jocmr5159. Epub 2024 Jun 18. J Clin Med Res. 2024. PMID: 39027810 Free PMC article.
-
Formalin-evoked pain triggers sex-specific behavior and spinal immune response.Sci Rep. 2023 Jun 12;13(1):9515. doi: 10.1038/s41598-023-36245-7. Sci Rep. 2023. PMID: 37308519 Free PMC article.
-
The influence of sex on neuroimmune communication, pain, and physiology.Biol Sex Differ. 2024 Oct 22;15(1):82. doi: 10.1186/s13293-024-00660-w. Biol Sex Differ. 2024. PMID: 39439003 Free PMC article. Review.
-
Sex differences in pain along the neuraxis.Neuropharmacology. 2022 Jun 1;210:109030. doi: 10.1016/j.neuropharm.2022.109030. Epub 2022 Mar 21. Neuropharmacology. 2022. PMID: 35331712 Free PMC article. Review.
References
-
- National Institutes of Health. Consideration of Sex as a Biological Variable in NIH-funded Research. National Institutes of Health; 2015:Notice Number: NOT-OD-15-102. https://grants.nih.gov/grants/guide/notice-files/not-od-15-102.html
-
- Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci. 2020;21(7):353-365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

