Epigenetics of Hepatic Insulin Resistance
- PMID: 34046015
- PMCID: PMC8147868
- DOI: 10.3389/fendo.2021.681356
Epigenetics of Hepatic Insulin Resistance
Abstract
Insulin resistance (IR) is largely recognized as a unifying feature that underlies metabolic dysfunction. Both lifestyle and genetic factors contribute to IR. Work from recent years has demonstrated that the epigenome may constitute an interface where different signals may converge to promote IR gene expression programs. Here, we review the current knowledge of the role of epigenetics in hepatic IR, focusing on the roles of DNA methylation and histone post-translational modifications. We discuss the broad epigenetic changes observed in the insulin resistant liver and its associated pathophysiological states and leverage on the wealth of 'omics' studies performed to discuss efforts in pinpointing specific loci that are disrupted by these changes. We envision that future studies, with increased genomic resolution and larger cohorts, will further the identification of biomarkers of early onset hepatic IR and assist the development of targeted interventions. Furthermore, there is growing evidence to suggest that persistent epigenetic marks may be acquired over prolonged exposure to disease or deleterious exposures, highlighting the need for preventative medicine and long-term lifestyle adjustments to avoid irreversible or long-term alterations in gene expression.
Keywords: NAFLD (non-alcoholic fatty liver disease); epigenetics (DNA methylation, histone modifications); insulin resistance; liver; type 2 diabetes.
Copyright © 2021 Maude, Sanchez-Cabanillas and Cebola.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
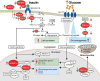
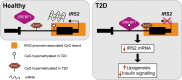
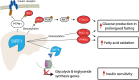
Similar articles
-
Epigenetics in non-alcoholic fatty liver disease.Mol Aspects Med. 2017 Apr;54:78-88. doi: 10.1016/j.mam.2016.11.008. Epub 2016 Nov 23. Mol Aspects Med. 2017. PMID: 27889327 Review.
-
Increased Hepatic PDGF-AA Signaling Mediates Liver Insulin Resistance in Obesity-Associated Type 2 Diabetes.Diabetes. 2018 Jul;67(7):1310-1321. doi: 10.2337/db17-1539. Epub 2018 May 4. Diabetes. 2018. PMID: 29728363 Clinical Trial.
-
Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: impact of liver methylation of the peroxisome proliferator-activated receptor γ coactivator 1α promoter.Hepatology. 2010 Dec;52(6):1992-2000. doi: 10.1002/hep.23927. Epub 2010 Oct 1. Hepatology. 2010. PMID: 20890895
-
Epigenetic Markers and Microbiota/Metabolite-Induced Epigenetic Modifications in the Pathogenesis of Obesity, Metabolic Syndrome, Type 2 Diabetes, and Non-alcoholic Fatty Liver Disease.Curr Diab Rep. 2019 May 1;19(6):31. doi: 10.1007/s11892-019-1151-4. Curr Diab Rep. 2019. PMID: 31044315 Free PMC article. Review.
-
Potential epigenetic mechanism in non-alcoholic Fatty liver disease.Int J Mol Sci. 2015 Mar 5;16(3):5161-79. doi: 10.3390/ijms16035161. Int J Mol Sci. 2015. PMID: 25751727 Free PMC article. Review.
Cited by
-
Epigenetic Regulation in Lean Nonalcoholic Fatty Liver Disease.Int J Mol Sci. 2023 Aug 16;24(16):12864. doi: 10.3390/ijms241612864. Int J Mol Sci. 2023. PMID: 37629043 Free PMC article. Review.
-
The Role of Insulin Resistance in Fueling NAFLD Pathogenesis: From Molecular Mechanisms to Clinical Implications.J Clin Med. 2022 Jun 24;11(13):3649. doi: 10.3390/jcm11133649. J Clin Med. 2022. PMID: 35806934 Free PMC article. Review.
-
Genetics, epigenetics and transgenerational transmission of obesity in children.Front Endocrinol (Lausanne). 2022 Nov 14;13:1006008. doi: 10.3389/fendo.2022.1006008. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36452324 Free PMC article. Review.
-
Epigenetics in Obesity and Diabetes Mellitus: New Insights.Nutrients. 2023 Feb 4;15(4):811. doi: 10.3390/nu15040811. Nutrients. 2023. PMID: 36839169 Free PMC article. Review.
-
Hepatoprotective effect of protocatechuic acid against type 2 diabetes-induced liver injury.Pharm Biol. 2023 Dec;61(1):737-745. doi: 10.1080/13880209.2023.2181359. Pharm Biol. 2023. PMID: 37129023 Free PMC article.
References
-
- Alberti K, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. . Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation (2009) 120(16):1640–5. 10.1161/CIRCULATIONAHA.109.192644 - DOI - PubMed
-
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. . Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results From the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract (2019) 157:107843. 10.1016/j.diabres.2019.107843 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

