Amphiphilic Iodine(III) Reagents for the Lipophilization of Peptides in Water
- PMID: 34038604
- PMCID: PMC8456932
- DOI: 10.1002/anie.202106458
Amphiphilic Iodine(III) Reagents for the Lipophilization of Peptides in Water
Abstract
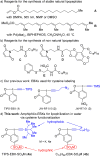
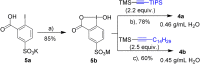
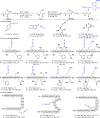
We report the functionalization of cysteine residues with lipophilic alkynes bearing a silyl group or an alkyl chain using amphiphilic ethynylbenziodoxolone reagents (EBXs). The reactions were carried out in buffer (pH 6 to 9), without organic co-solvent or removal of oxygen, either at 37 °C or room temperature. The transformation led to a significant increase of peptide lipophilicity and worked for aromatic thiols, homocysteine, cysteine, and peptides containing 4 to 18 amino acids. His6 -Cys-Ubiquitin was also alkynylated under physiological conditions. Under acidic conditions, the thioalkynes were converted into thioesters, which could be cleaved in the presence of hydroxylamine.
Keywords: amphiphilic reagents; hypervalent iodine; lipidation; lipopeptide; ubiquitin.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
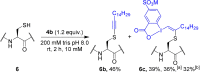

Similar articles
-
Peptide-Hypervalent Iodine Reagent Chimeras: Enabling Peptide Functionalization and Macrocyclization.Angew Chem Int Ed Engl. 2023 Aug 14;62(33):e202306036. doi: 10.1002/anie.202306036. Epub 2023 Jul 7. Angew Chem Int Ed Engl. 2023. PMID: 37311172
-
Cyclic Hypervalent Iodine Reagents: Enabling Tools for Bond Disconnection via Reactivity Umpolung.Acc Chem Res. 2018 Dec 18;51(12):3212-3225. doi: 10.1021/acs.accounts.8b00468. Epub 2018 Nov 28. Acc Chem Res. 2018. PMID: 30485071
-
Cys-Cys and Cys-Lys Stapling of Unprotected Peptides Enabled by Hypervalent Iodine Reagents.Angew Chem Int Ed Engl. 2021 Apr 12;60(16):9022-9031. doi: 10.1002/anie.202014511. Epub 2021 Mar 8. Angew Chem Int Ed Engl. 2021. PMID: 33450121 Free PMC article.
-
Hypervalent Iodine-Mediated Late-Stage Peptide and Protein Functionalization.Angew Chem Int Ed Engl. 2022 Feb 7;61(7):e202112287. doi: 10.1002/anie.202112287. Epub 2021 Dec 8. Angew Chem Int Ed Engl. 2022. PMID: 34674359 Free PMC article. Review.
-
Hypervalent Iodine Reagents in Palladium-Catalyzed Oxidative Cross-Coupling Reactions.Front Chem. 2020 Sep 29;8:705. doi: 10.3389/fchem.2020.00705. eCollection 2020. Front Chem. 2020. PMID: 33134246 Free PMC article. Review.
Cited by
-
Functionalized quinolizinium-based fluorescent reagents for modification of cysteine-containing peptides and proteins.RSC Adv. 2022 Feb 22;12(10):6248-6254. doi: 10.1039/d1ra08329e. eCollection 2022 Feb 16. RSC Adv. 2022. PMID: 35424586 Free PMC article.
-
Recent progress in alkynylation with hypervalent iodine reagents.Chem Commun (Camb). 2023 Feb 7;59(12):1589-1604. doi: 10.1039/d2cc06168f. Chem Commun (Camb). 2023. PMID: 36656618 Free PMC article. Review.
-
Selective editing of a peptide skeleton via C-N bond formation at N-terminal aliphatic side chains.Chem Sci. 2022 Nov 22;13(48):14382-14386. doi: 10.1039/d2sc04909k. eCollection 2022 Dec 14. Chem Sci. 2022. PMID: 36545141 Free PMC article.
-
Electrochemical Synthesis of Dimeric λ3-Bromane: Platform for Hypervalent Bromine(III) Compounds.Org Lett. 2023 Mar 31;25(12):2047-2052. doi: 10.1021/acs.orglett.3c00405. Epub 2023 Mar 21. Org Lett. 2023. PMID: 36944352 Free PMC article.
-
A Vitamin B2-Photocatalysed Approach to Methionine Analogues.Angew Chem Weinheim Bergstr Ger. 2022 Dec 12;134(50):e202212158. doi: 10.1002/ange.202212158. Epub 2022 Nov 10. Angew Chem Weinheim Bergstr Ger. 2022. PMID: 38505624 Free PMC article.
References
-
- None
-
- Castanho M., Santos N. C., Peptide Drug Discovery and Development, Wiley-VCH, Weinheim, 2011;
-
- Craik D. J., Fairlie D. P., Liras S., Price D., Chem. Biol. Drug Des. 2013, 81, 136; - PubMed
-
- Dunn B. M., Peptide Chemistry and Drug Design, Wiley, Hoboken, 2015;
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

