Propofol maintains Th17/Treg cell balance and reduces inflammation in rats with traumatic brain injury via the miR‑145‑3p/NFATc2/NF‑κB axis
- PMID: 34036377
- PMCID: PMC8148094
- DOI: 10.3892/ijmm.2021.4968
Propofol maintains Th17/Treg cell balance and reduces inflammation in rats with traumatic brain injury via the miR‑145‑3p/NFATc2/NF‑κB axis
Abstract
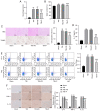
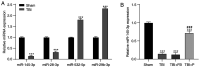
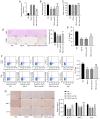
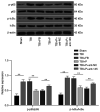
Propofol is a commonly used intravenous anesthetic. The aim of the study was to examine the mechanism of propofol in traumatic brain injury (TBI) by regulating interleukin (IL)‑17 activity and maintaining the Th17/Treg balance. A rat model with moderate TBI was established using the weight‑drop method. Rats with TBI were regularly injected with propofol and their brain injuries were monitored. The peripheral blood of rats was collected to measure the Th17/Treg ratio. MicroRNA (miR)‑145‑3p expression was detected in the brain tissues of rats and antagomiR‑145‑3p was injected into the lateral ventricles of their brains to verify the effect of miR‑145‑3p on brain injury. The downstream target of miR‑145‑3p was predicted. The targeting relationship between miR‑145‑3p and nuclear factor of activated T cells c2 (NFATc2) was confirmed. NFATC2 expression and phosphorylation of NF‑κB pathway‑related proteins were measured. Propofol alleviated brain injury in rats with TBI and maintained the Th17/Treg balance. Propofol upregulated miR‑145‑3p expression in rat brains, while the inhibition of miR‑145‑3p reversed the effect of propofol on brain injury. A binding relationship was observed between miR‑145‑3p and NFATc2. Furthermore, propofol decreased the phosphorylation of p65 and IκBα, and inhibited activation of the NF‑κB pathway in the brains of rats with TBI. In conclusion, propofol maintained Th17/Treg balance and reduced inflammation in the rats with TBI via the miR‑145‑3p/NFATc2/NF‑κB axis.
Keywords: NF‑κB; Th17/Treg; inflammation; microRNA‑145‑3p; nuclear factor of activated T cells c2; propofol; traumatic brain injury.
Conflict of interest statement
All authors declare that they have no competing interests.
Figures

Similar articles
-
Umbilical cord mesenchymal stem cells promote neurological repair after traumatic brain injury through regulating Treg/Th17 balance.Brain Res. 2022 Jan 15;1775:147711. doi: 10.1016/j.brainres.2021.147711. Epub 2021 Nov 15. Brain Res. 2022. PMID: 34793756
-
Quercetin protects mouse liver against triptolide-induced hepatic injury by restoring Th17/Treg balance through Tim-3 and TLR4-MyD88-NF-κB pathway.Int Immunopharmacol. 2017 Dec;53:73-82. doi: 10.1016/j.intimp.2017.09.026. Epub 2017 Oct 15. Int Immunopharmacol. 2017. PMID: 29040945
-
Ulinastatin ameliorates acute kidney injury induced by crush syndrome inflammation by modulating Th17/Treg cells.Int Immunopharmacol. 2020 Apr;81:106265. doi: 10.1016/j.intimp.2020.106265. Epub 2020 Feb 7. Int Immunopharmacol. 2020. PMID: 32044661
-
Nuclear factor-κB in immunity and inflammation: the Treg and Th17 connection.Adv Exp Med Biol. 2012;946:207-21. doi: 10.1007/978-1-4614-0106-3_12. Adv Exp Med Biol. 2012. PMID: 21948370 Review.
-
Th17 and regulatory T cell balance in autoimmune and inflammatory diseases.Autoimmun Rev. 2014 Jun;13(6):668-77. doi: 10.1016/j.autrev.2013.12.004. Epub 2014 Jan 11. Autoimmun Rev. 2014. PMID: 24418308 Review.
Cited by
-
Research progress of neuroinflammation-related cells in traumatic brain injury: A review.Medicine (Baltimore). 2023 Jun 23;102(25):e34009. doi: 10.1097/MD.0000000000034009. Medicine (Baltimore). 2023. PMID: 37352020 Free PMC article. Review.
-
Role of regulatory non-coding RNAs in traumatic brain injury.Neurochem Int. 2024 Jan;172:105643. doi: 10.1016/j.neuint.2023.105643. Epub 2023 Nov 24. Neurochem Int. 2024. PMID: 38007071 Free PMC article. Review.
-
A Clinical Study on the Brain Protection Effect of Propofol Anesthesia on Patients Undergoing Acute Craniocerebral Trauma Surgery Based on Blockchain.J Healthc Eng. 2022 Apr 9;2022:6111543. doi: 10.1155/2022/6111543. eCollection 2022. J Healthc Eng. 2022. Retraction in: J Healthc Eng. 2023 Oct 4;2023:9761651. doi: 10.1155/2023/9761651. PMID: 35437464 Free PMC article. Retracted.
-
miR‑146a‑5p protects against renal injury in MRL/lpr mice via improvement of the Treg/Th17 imbalance by targeting the TRAF6/NF‑κB axis.Exp Ther Med. 2022 Nov 22;25(1):21. doi: 10.3892/etm.2022.11720. eCollection 2023 Jan. Exp Ther Med. 2022. PMID: 38895650 Free PMC article.
-
Efficacy and safety of remimazolam tosilate versus propofol in patients undergoing day surgery: a prospective randomized controlled trial.BMC Anesthesiol. 2023 May 26;23(1):182. doi: 10.1186/s12871-023-02092-2. BMC Anesthesiol. 2023. PMID: 37237331 Free PMC article. Clinical Trial.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

