Cutaneous and ocular rosacea: Common and specific physiopathogenic mechanisms and study models
- PMID: 34035646
- PMCID: PMC8131178
Cutaneous and ocular rosacea: Common and specific physiopathogenic mechanisms and study models
Abstract
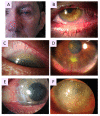
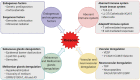
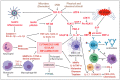
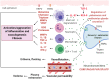
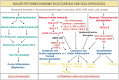
Rosacea is a chronic inflammatory disease that affects the face skin. It is clinically classified into the following four subgroups depending on its location and severity: erythematotelangiectatic, papulopustular, phymatous, and ocular. Rosacea is a multifactorial disease triggered by favoring factors, the pathogenesis of which remains imperfectly understood. Recognized mechanisms include the innate immune system, with the implication of Toll-like receptors (TLRs) and cathelicidins; neurovascular deregulation involving vascular endothelial growth factor (VEGF), transient receptor potential (TRP) ion channels, and neuropeptides; and dysfunction of skin sebaceous glands and ocular meibomian glands. Microorganisms, genetic predisposition, corticosteroid treatment, and ultraviolet B (UVB) radiation are favoring factors. In this paper, we review the common and specific molecular mechanisms involved in the pathogenesis of cutaneous and ocular rosacea and discuss laboratory and clinical studies, as well as experimental models.
Copyright © 2021 Molecular Vision.
Figures
Similar articles
-
New insights into rosacea pathophysiology: a review of recent findings.J Am Acad Dermatol. 2013 Dec;69(6 Suppl 1):S15-26. doi: 10.1016/j.jaad.2013.04.045. J Am Acad Dermatol. 2013. PMID: 24229632 Review.
-
Update on the pathogenesis and management of ocular rosacea: an interdisciplinary review.Eur J Ophthalmol. 2021 Jan;31(1):22-33. doi: 10.1177/1120672120937252. Epub 2020 Jun 25. Eur J Ophthalmol. 2021. PMID: 32586107 Review.
-
Rosacea: Molecular Mechanisms and Management of a Chronic Cutaneous Inflammatory Condition.Int J Mol Sci. 2016 Sep 15;17(9):1562. doi: 10.3390/ijms17091562. Int J Mol Sci. 2016. PMID: 27649161 Free PMC article. Review.
-
Rosacea: part I. Introduction, categorization, histology, pathogenesis, and risk factors.J Am Acad Dermatol. 2015 May;72(5):749-58; quiz 759-60. doi: 10.1016/j.jaad.2014.08.028. J Am Acad Dermatol. 2015. PMID: 25890455 Review.
-
Rosacea: an update.Dermatology. 2005;210(2):100-8. doi: 10.1159/000082564. Dermatology. 2005. PMID: 15724091 Review.
Cited by
-
Rosacea, microbiome and probiotics: the gut-skin axis.Front Microbiol. 2024 Jan 8;14:1323644. doi: 10.3389/fmicb.2023.1323644. eCollection 2023. Front Microbiol. 2024. PMID: 38260914 Free PMC article. Review.
-
The Multifold Etiologies of Limbal Stem Cell Deficiency: A Comprehensive Review on the Etiologies and Additional Treatment Options for Limbal Stem Cell Deficiency.J Clin Med. 2023 Jun 30;12(13):4418. doi: 10.3390/jcm12134418. J Clin Med. 2023. PMID: 37445454 Free PMC article. Review.
-
Interaction Between Blood Vasculatures and Lymphatic Vasculatures During Inflammation.J Inflamm Res. 2023 Aug 4;16:3271-3281. doi: 10.2147/JIR.S414891. eCollection 2023. J Inflamm Res. 2023. PMID: 37560514 Free PMC article. Review.
-
Toll-like receptor-4 expression and oxidative stress in ocular rosacea.Mol Vis. 2023 Dec 26;29:357-364. eCollection 2023. Mol Vis. 2023. PMID: 38577560 Free PMC article.
-
The role of macrophages in rosacea: implications for targeted therapies.Front Immunol. 2023 Aug 24;14:1211953. doi: 10.3389/fimmu.2023.1211953. eCollection 2023. Front Immunol. 2023. PMID: 37691916 Free PMC article. Review.
References
-
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: A review of recent findings. J Am Acad Dermatol. 2013;69(Supplement 1):S15–26. - PubMed
-
- Steinhoff M, Schmelz M, Schauber J. Facial Erythema of Rosacea - Aetiology, Different Pathophysiologies and Treatment Options. Acta Derm Venereol. 2016;96:579–86. - PubMed
-
- Gallo RL, Granstein RD, Kang S, Mannis M, Steinhoff M, Tan J, Thiboutot D. Standard classification and pathophysiology of rosacea: The 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148–55. - PubMed
-
- Wilkin J, Dahl M, Detmar M, Drake L, Feinstein A, Odom R, Powell F. Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584–7. - PubMed
-
- Gold LM, Draelos ZD. New and Emerging Treatments for Rosacea. Am J Clin Dermatol. 2015;16:457–61. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

