Inflammatory signals are sufficient to elicit TOX expression in mouse and human CD8+ T cells
- PMID: 34032638
- PMCID: PMC8410038
- DOI: 10.1172/jci.insight.150744
Inflammatory signals are sufficient to elicit TOX expression in mouse and human CD8+ T cells
Abstract
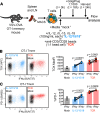
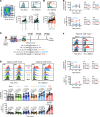
T cell receptor (TCR) stimulation leads to the expression of the transcription factor thymocyte selection-associated high-mobility group box (TOX). Prolonged TCR signaling, such as encountered during chronic infections or in tumors, leads to sustained TOX expression, which is required for the induction of a state of exhaustion or dysfunction. Although CD8+ memory T (Tmem) cells in mice typically do not express TOX at steady state, some human Tmem cells express TOX but appear fully functional. This seeming discrepancy between mouse and human T cells has led to the speculation that TOX is differentially regulated between these species, which could complicate the interpretation of preclinical mouse model studies. We report here that, similar to TCR-mediated signals, inflammatory cytokines are also sufficient to increase TOX expression in human and mouse Tmem cells. Thus, TOX expression is controlled by the environment, which provides an explanation for the different TOX expression patterns encountered in T cells isolated from specific pathogen-free laboratory mice versus humans. Finally, we report that TOX is not necessary for cytokine-driven expression of programmed cell death 1. Overall, our data highlight that the mechanisms regulating TOX expression are conserved across species and indicate that TOX expression reflects a T cell's activation state and does not necessarily correlate with T cell dysfunction.
Keywords: Immunology; Inflammation; T cells.
Conflict of interest statement
Figures





Similar articles
-
TOX promotes the exhaustion of antitumor CD8+ T cells by preventing PD1 degradation in hepatocellular carcinoma.J Hepatol. 2019 Oct;71(4):731-741. doi: 10.1016/j.jhep.2019.05.015. Epub 2019 Jun 5. J Hepatol. 2019. PMID: 31173813
-
TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection.Nature. 2019 Jul;571(7764):265-269. doi: 10.1038/s41586-019-1326-9. Epub 2019 Jun 17. Nature. 2019. PMID: 31207605
-
TOX is a critical regulator of tumour-specific T cell differentiation.Nature. 2019 Jul;571(7764):270-274. doi: 10.1038/s41586-019-1324-y. Epub 2019 Jun 17. Nature. 2019. PMID: 31207604 Free PMC article.
-
Role, function and regulation of the thymocyte selection-associated high mobility group box protein in CD8+ T cell exhaustion.Immunol Lett. 2021 Jan;229:1-7. doi: 10.1016/j.imlet.2020.11.004. Epub 2020 Nov 11. Immunol Lett. 2021. PMID: 33186634 Review.
-
The TOX subfamily: all-round players in the immune system.Clin Exp Immunol. 2022 Jun 23;208(3):268-280. doi: 10.1093/cei/uxac037. Clin Exp Immunol. 2022. PMID: 35485425 Free PMC article. Review.
Cited by
-
The TOX-RAGE axis mediates inflammatory activation and lung injury in severe pulmonary infectious diseases.Proc Natl Acad Sci U S A. 2024 Jun 25;121(26):e2319322121. doi: 10.1073/pnas.2319322121. Epub 2024 Jun 20. Proc Natl Acad Sci U S A. 2024. PMID: 38900789 Free PMC article.
-
Defining and targeting patterns of T cell dysfunction in inborn errors of immunity.Front Immunol. 2022 Sep 14;13:932715. doi: 10.3389/fimmu.2022.932715. eCollection 2022. Front Immunol. 2022. PMID: 36189259 Free PMC article. Review.
-
Phenotype and fate of liver-resident CD8 T cells during acute and chronic hepacivirus infection.PLoS Pathog. 2023 Oct 9;19(10):e1011697. doi: 10.1371/journal.ppat.1011697. eCollection 2023 Oct. PLoS Pathog. 2023. PMID: 37812637 Free PMC article.
-
Tumor microenvironmental signals reshape chromatin landscapes to limit the functional potential of exhausted T cells.Sci Immunol. 2022 Aug 5;7(74):eabj9123. doi: 10.1126/sciimmunol.abj9123. Epub 2022 Aug 5. Sci Immunol. 2022. PMID: 35930654 Free PMC article.
-
Fundamentals to therapeutics: Epigenetic modulation of CD8+ T Cell exhaustion in the tumor microenvironment.Front Cell Dev Biol. 2023 Jan 4;10:1082195. doi: 10.3389/fcell.2022.1082195. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36684449 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

