Juvenile Selenium Deficiency Impairs Cognition, Sensorimotor Gating, and Energy Homeostasis in Mice
- PMID: 34026810
- PMCID: PMC8138326
- DOI: 10.3389/fnut.2021.667587
Juvenile Selenium Deficiency Impairs Cognition, Sensorimotor Gating, and Energy Homeostasis in Mice
Abstract
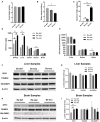
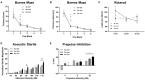
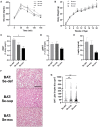
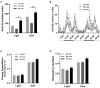
Selenium (Se) is an essential micronutrient of critical importance to mammalian life. Its biological effects are primarily mediated via co-translational incorporation into selenoproteins, as the unique amino acid, selenocysteine. These proteins play fundamental roles in redox signaling and includes the glutathione peroxidases and thioredoxin reductases. Environmental distribution of Se varies considerably worldwide, with concomitant effects on Se status in humans and animals. Dietary Se intake within a narrow range optimizes the activity of Se-dependent antioxidant enzymes, whereas both Se-deficiency and Se-excess can adversely impact health. Se-deficiency affects a significant proportion of the world's population, with hypothyroidism, cardiomyopathy, reduced immunity, and impaired cognition being common symptoms. Although relatively less prevalent, Se-excess can also have detrimental consequences and has been implicated in promoting both metabolic and neurodegenerative disease in humans. Herein, we sought to comprehensively assess the developmental effects of both Se-deficiency and Se-excess on a battery of neurobehavioral and metabolic tests in mice. Se-deficiency elicited deficits in cognition, altered sensorimotor gating, and increased adiposity, while Se-excess was surprisingly beneficial.
Keywords: cognition; energy metabolism; neurodevelopment; selenium; sensorimotor gating.
Copyright © 2021 Kilonzo, Sasuclark, Torres, Coyle, Pilat, Williams and Pitts.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Selenium, Selenoproteins and Viral Infection.Nutrients. 2019 Sep 4;11(9):2101. doi: 10.3390/nu11092101. Nutrients. 2019. PMID: 31487871 Free PMC article. Review.
-
Selenium, selenoproteins and human health: a review.Public Health Nutr. 2001 Apr;4(2B):593-9. doi: 10.1079/phn2001143. Public Health Nutr. 2001. PMID: 11683552 Review.
-
The role of selenium in thyroid autoimmunity and cancer.Thyroid. 2006 May;16(5):455-60. doi: 10.1089/thy.2006.16.455. Thyroid. 2006. PMID: 16756467
-
Selenium in Complicated Pregnancy. A Review.Adv Clin Chem. 2018;86:157-178. doi: 10.1016/bs.acc.2018.05.004. Epub 2018 Jul 31. Adv Clin Chem. 2018. PMID: 30144839 Review.
-
Dietary selenium deficiency or selenomethionine excess drastically alters organ selenium contents without altering the expression of most selenoproteins in mice.J Nutr Biochem. 2019 Jul;69:120-129. doi: 10.1016/j.jnutbio.2019.03.020. Epub 2019 Apr 8. J Nutr Biochem. 2019. PMID: 31078905
Cited by
-
Critical Role of Maternal Selenium Nutrition in Neurodevelopment: Effects on Offspring Behavior and Neuroinflammatory Profile.Nutrients. 2022 Apr 28;14(9):1850. doi: 10.3390/nu14091850. Nutrients. 2022. PMID: 35565817 Free PMC article.
-
Selenium Intake and Postnatal Depression-A Short Review.Nutrients. 2024 Jun 18;16(12):1926. doi: 10.3390/nu16121926. Nutrients. 2024. PMID: 38931280 Free PMC article. Review.
References
-
- Ikemoto T, Kunito T, Tanaka H, Baba N, Miyazaki N, Tanabe S. Detoxification mechanism of heavy metals in marine mammals and seabirds: interaction of selenium with mercury, silver, copper, zinc, and cadmium in liver. Arch Environ Contam Toxicol. (2004) 47:402–13. 10.1007/s00244-004-3188-9 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

