Instruments to measure outcomes of post-intensive care syndrome in outpatient care settings - Results of an expert consensus and feasibility field test
- PMID: 34025756
- PMCID: PMC8120565
- DOI: 10.1177/1751143720923597
Instruments to measure outcomes of post-intensive care syndrome in outpatient care settings - Results of an expert consensus and feasibility field test
Abstract
Background: There is no consensus on the instruments for diagnosis of post-intensive care syndrome (PICS). We present a proposal for a set of outcome measurement instruments of PICS in outpatient care.
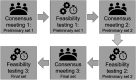
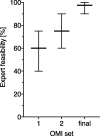
Methods: We conducted a three-round, semi-structured consensus-seeking process with medical experts, followed each by exploratory feasibility investigations with intensive care unit survivors (n1 = 5; n2 = 5; n3 = 7). Fourteen participants from nine stakeholder groups participated in the first and second consensus meeting. In the third consensus meeting, a core group of six clinical researchers refined the final outcome measurement instrument set proposal.
Results: We suggest an outcome measurement instrument set used in a two-step process. First step: Screening with brief tests covering PICS domains of (1) mental health (Patient Health Questionnaire-4 (PHQ-4)), (2) cognition (MiniCog, Animal Naming), (3) physical function (Timed Up-and-Go (TUG), handgrip strength), and (4) health-related quality of life (HRQoL) (EQ-5D-5L). Single items measure subjective health before and after the intensive care unit stay. If patients report new or worsened health problems after intensive care unit discharge and show relevant impairment in at least one of the screening tests, a second extended assessment follows: (1) Mental health (Patient Health Questionnaire-8 (PHQ-8), Generalized Anxiety Disorder Scale-7 (GAD-7), Impact of Event Scale - revised (IES-R)); (2) cognition (Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), Trail Making Test (TMT) A and B); (3) physical function (2-Minute Walk Test (2-MWT), handgrip strength, Short Physical Performance Battery (SPPB)); and (4) HRQoL (EQ-5D-5L, 12-Item WHO Disability Assessment Schedule (WHODAS 2.0)).
Conclusions: We propose an outcome measurement instrument set used in a two-step measurement of PICS, combining performance-based and patient-reported outcome measures. First-step screening is brief, free-of-charge, and easily applicable by health care professionals across different sectors. If indicated, specialized healthcare providers can perform the extended, second-step assessment. Usage of the first-step screening of our suggested outcome measurement instrument set in outpatient clinics with subsequent transfer to specialists is recommended for all intensive care unit survivors. This may increase awareness and reduce the burden of PICS.
Trial registration: This study was registered at ClinicalTrials.gov (Identifier: NCT04175236; first posted 22 November 2019).
Keywords: Post-intensive care syndrome; cognition; critical care; intensive care medicine; intensive care unit; mental health; physical function; survivors.
© The Intensive Care Society 2020.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CDS reports grants from Gemeinsamer Bundesausschuss (G-BA)/Federal Joint Committee, during the conduct of the study; grants from Aridis Pharmaceutical Inc., grants from B Braun Melsungen AG, grants from Drägerwerk AG & Co KGaA, grants from Grünenthal GmbH, grants from Infectopharm GmbH, grants from Sedana Medical Ltd, grants from Deutsche Forschungsgemeinschaft/German Research Society, grants from Deutsches Zentrum für Luft- und Raumfahrt e.V. (DLR)/German Aerospace Center, grants from Einstein Stiftung Berlin/Einstein Foundation Berlin, grants from the European Society of Anaesthesiology, grants from Gemeinsamer Bundesausschuss (G-BA)/Federal Joint Committee, grants from Inneruniversitäre Forschungsförderung/Inner University Grants, grants from Projektträger im DLR/Project Management Agency, grants from Stifterverband/Non-Profit Society Promoting Science and Education, grants from WHOCC, grants from Baxter Deutschland GmbH, grants from Biotest AG, grants from Cytosorbents Europe GmbH, grants from Edwards Lifesciences Germany GmbH, grants from Fresenius Medical Care, grants from Grünenthal GmbH, grants from Masimo Europe Ltd, grants from Medtronic GmbH, grants from Pfizer Pharma PFE GmbH, personal fees from Georg Thieme Verlag, grants from Dr. F. Köhler Chemie GmbH, grants from Sintetica GmbH, grants from the European Commission, grants from Stifterverband für die Deutsche Wissenschaft e.V./Philips, grants from Stiftung Charité, outside the submitted work; in addition, CDS has a patent 10 2014 215 211.9 pending, a patent Application No. PCT/EP2015/067730 pending to Graft Gesellschaft von Architekten mbH, and a patent Application No. PCT/EP2015/067731 pending to Graft Gesellschaft von Architekten mbH. HK, NP, TG, SR, JJG, KSc, KSt, JC, JKr, JKi, CD, SP, CA, RB, UM, SWC and MG declare that there is no conflict of interest. CH reports grants to her institution from the German Federal Ministry of Education and Research (BMBF) via the Center for Sepsis Control and Care Jena, the German Innovations Funds/Federal Joint Committee, and the European Society of Intensive Care Medicine. BW reports personal fees from Orion Pharma Ltd. and personal fees from Dr. F. Köhler Chemie GmbH, outside the submitted work.
Figures

Similar articles
-
Instruments to assess post-intensive care syndrome assessment: a scoping review and modified Delphi method study.Crit Care. 2023 Nov 7;27(1):430. doi: 10.1186/s13054-023-04681-6. Crit Care. 2023. PMID: 37936249 Free PMC article. Review.
-
Core Outcome Measures for Clinical Research in Acute Respiratory Failure Survivors. An International Modified Delphi Consensus Study.Am J Respir Crit Care Med. 2017 Nov 1;196(9):1122-1130. doi: 10.1164/rccm.201702-0372OC. Am J Respir Crit Care Med. 2017. PMID: 28537429 Free PMC article.
-
Measures for the Core Outcome Set for Research Evaluating Interventions to Prevent and/or Treat Delirium in Critically Ill Adults: An International Consensus Study (Del-COrS).Crit Care Explor. 2023 Apr 3;5(4):e0884. doi: 10.1097/CCE.0000000000000884. eCollection 2023 Apr. Crit Care Explor. 2023. PMID: 37025304 Free PMC article.
-
Erratum.Mult Scler. 2016 Oct;22(12):NP9-NP11. doi: 10.1177/1352458515585718. Epub 2015 Jun 3. Mult Scler. 2016. PMID: 26041800
-
Society of Critical Care Medicine's International Consensus Conference on Prediction and Identification of Long-Term Impairments After Critical Illness.Crit Care Med. 2020 Nov;48(11):1670-1679. doi: 10.1097/CCM.0000000000004586. Crit Care Med. 2020. PMID: 32947467 Review.
Cited by
-
Effects on health-related quality of life of interventions affecting survival in critically ill patients: a systematic review.Crit Care. 2022 May 6;26(1):126. doi: 10.1186/s13054-022-03993-3. Crit Care. 2022. PMID: 35524315 Free PMC article. Review.
-
Reliability and Effectiveness of the Japanese Version of the Mobilization Quantification Score.Cureus. 2023 Aug 13;15(8):e43440. doi: 10.7759/cureus.43440. eCollection 2023 Aug. Cureus. 2023. PMID: 37711928 Free PMC article.
-
Functional outcome after interdisciplinary, acute rehabilitation in COVID-19 patients: a retrospective study.Eur Arch Psychiatry Clin Neurosci. 2024 Dec;274(8):1993-2001. doi: 10.1007/s00406-024-01862-4. Epub 2024 Jul 16. Eur Arch Psychiatry Clin Neurosci. 2024. PMID: 39012495 Free PMC article.
-
Core outcomes sets for studies evaluating critical illness and patient recovery.Curr Opin Crit Care. 2020 Oct;26(5):489-499. doi: 10.1097/MCC.0000000000000750. Curr Opin Crit Care. 2020. PMID: 32773613 Free PMC article. Review.
-
Post-Intensive Care Syndrome as a Burden for Patients and Their Caregivers: A Narrative Review.J Clin Med. 2024 Oct 2;13(19):5881. doi: 10.3390/jcm13195881. J Clin Med. 2024. PMID: 39407940 Free PMC article. Review.
References
-
- Elliott D, Davidson JE, Harvey MA, et al. Exploring the scope of post-intensive care syndrome therapy and care: engagement of non-critical care providers and survivors in a second stakeholders meeting. Crit Care Med 2014; 42: 2518–2526. - PubMed
-
- Iwashyna TJ. Survivorship will be the defining challenge of critical care in the 21st century. Ann Intern Med 2010; 153: 204–205. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
