Peripheral Injection of Tim-3 Antibody Attenuates VSV Encephalitis by Enhancing MHC-I Presentation
- PMID: 34025669
- PMCID: PMC8138436
- DOI: 10.3389/fimmu.2021.667478
Peripheral Injection of Tim-3 Antibody Attenuates VSV Encephalitis by Enhancing MHC-I Presentation
Abstract
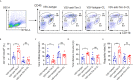
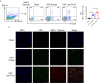
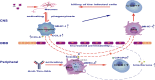
Viral encephalitis is the most common cause of encephalitis. It is responsible for high morbidity rates, permanent neurological sequelae, and even high mortality rates. The host immune response plays a critical role in preventing or clearing invading pathogens, especially when effective antiviral treatment is lacking. However, due to blockade of the blood-brain barrier, it remains unclear how peripheral immune cells contribute to the fight against intracerebral viruses. Here, we report that peripheral injection of an antibody against human Tim-3, an immune checkpoint inhibitor widely expressed on immune cells, markedly attenuated vesicular stomatitis virus (VSV) encephalitis, marked by decreased mortality and improved neuroethology in mice. Peripheral injection of Tim-3 antibody enhanced the recruitment of immune cells to the brain, increased the expression of major histocompatibility complex-I (MHC-I) on macrophages, and as a result, promoted the activation of VSV-specific CD8+ T cells. Depletion of macrophages abolished the peripheral injection-mediated protection against VSV encephalitis. Notably, for the first time, we found a novel post-translational modification of MHC-I by Tim-3, wherein, by enhancing the expression of MARCH9, Tim-3 promoted the proteasome-dependent degradation of MHC-I via K48-linked ubiquitination in macrophages. These results provide insights into the immune response against intracranial infections; thus, manipulating the peripheral immune cells with Tim-3 antibody to fight viruses in the brain may have potential applications for combating viral encephalitis.
Keywords: Tim-3; encephalitis; macrophages; major histocompatibility complex-I; ubiquitination; vesicular stomatitis virus.
Copyright © 2021 Li, Tang, Hou, Wang, Gao, Dou, Mo, Hao, Gao, Li, Dong, Zhang, Shen, Wang and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Ubiquitination and degradation of NF90 by Tim-3 inhibits antiviral innate immunity.Elife. 2021 Jun 10;10:e66501. doi: 10.7554/eLife.66501. Elife. 2021. PMID: 34110282 Free PMC article.
-
Peripheral immunization blocks lethal actions of vesicular stomatitis virus within the brain.J Virol. 2009 Nov;83(22):11540-9. doi: 10.1128/JVI.02558-08. Epub 2009 Sep 2. J Virol. 2009. PMID: 19726512 Free PMC article.
-
Upon intranasal vesicular stomatitis virus infection, astrocytes in the olfactory bulb are important interferon Beta producers that protect from lethal encephalitis.J Virol. 2015 Mar;89(5):2731-8. doi: 10.1128/JVI.02044-14. Epub 2014 Dec 24. J Virol. 2015. PMID: 25540366 Free PMC article.
-
Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus.Nature. 2010 Jun 24;465(7301):1079-83. doi: 10.1038/nature09118. Nature. 2010. PMID: 20577213 Free PMC article.
-
Advances in viral encephalitis: Viral transmission, host immunity, and experimental animal models.Zool Res. 2023 May 18;44(3):525-542. doi: 10.24272/j.issn.2095-8137.2023.025. Zool Res. 2023. PMID: 37073800 Free PMC article. Review.
Cited by
-
LILRB2 promotes immune escape in breast cancer cells via enhanced HLA-A degradation.Cell Oncol (Dordr). 2024 Oct;47(5):1679-1696. doi: 10.1007/s13402-024-00947-5. Epub 2024 Apr 24. Cell Oncol (Dordr). 2024. PMID: 38656573
-
Tim-3 Relieves Experimental Autoimmune Encephalomyelitis by Suppressing MHC-II.Front Immunol. 2022 Jan 13;12:770402. doi: 10.3389/fimmu.2021.770402. eCollection 2021. Front Immunol. 2022. PMID: 35095844 Free PMC article.
-
Pediatric glioma immune profiling identifies TIM3 as a therapeutic target in BRAF fusion pilocytic astrocytoma.J Clin Invest. 2024 Aug 13;134(19):e177413. doi: 10.1172/JCI177413. J Clin Invest. 2024. PMID: 39137048 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

