Anti-Inflammatory Effects of Dimethyl Fumarate in Microglia via an Autophagy Dependent Pathway
- PMID: 34025399
- PMCID: PMC8137969
- DOI: 10.3389/fphar.2021.612981
Anti-Inflammatory Effects of Dimethyl Fumarate in Microglia via an Autophagy Dependent Pathway
Abstract
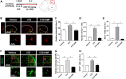
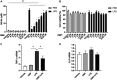
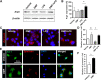
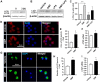
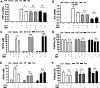
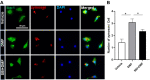
Dimethyl fumarate (DMF), which has been approved by the Food and Drug Administration for the treatment of relapsing-remitting multiple sclerosis, is considered to exert anti-inflammatory and antioxidant effects. Microglia maintain homeostasis in the central nervous system and play a key role in neuroinflammation, while autophagy controls numerous fundamental biological processes, including pathogen removal, cytokine production, and clearance of toxic aggregates. However, the role of DMF in autophagy induction and the relationship of this effect with its anti-inflammatory functions in microglia are not well known. In the present study, we investigated whether DMF inhibited neuroinflammation and induced autophagy in microglia. First, we confirmed the anti-neuroinflammatory effect of DMF in mice with streptozotocin-induced diabetic neuropathy. Next, we used in vitro models including microglial cell lines and primary microglial cells to examine the anti-inflammatory and neuroprotective effects of DMF. We found that DMF significantly inhibited nitric oxide and proinflammatory cytokine production in lipopolysaccharide-stimulated microglia and induced the switch of microglia to the M2 state. In addition, DMF treatment increased the expression levels of autophagy markers including microtubule-associated protein light chain 3 (LC3) and autophagy-related protein 7 (ATG7) and the formation of LC3 puncta in microglia. The anti-inflammatory effect of DMF in microglia was significantly reduced by pretreatment with autophagy inhibitors. These data suggest that DMF leads to the induction of autophagy in microglia and that its anti-inflammatory effects are partially mediated through an autophagy-dependent pathway.
Keywords: DMF; anti-inflammation; autophagy; microglia; neuroinflammation.
Copyright © 2021 Lee, Gupta, Park, Yang and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Dimethyl fumarate attenuates reactive microglia and long-term memory deficits following systemic immune challenge.J Neuroinflammation. 2018 Mar 29;15(1):100. doi: 10.1186/s12974-018-1125-5. J Neuroinflammation. 2018. PMID: 29598822 Free PMC article.
-
Dimethyl Fumarate Reduces Microglia Functional Response to Tissue Damage and Favors Brain Iron Homeostasis.Neuroscience. 2020 Jul 15;439:241-254. doi: 10.1016/j.neuroscience.2019.10.041. Epub 2019 Nov 15. Neuroscience. 2020. PMID: 31738884
-
Lipopolysaccharide induces neuroinflammation in microglia by activating the MTOR pathway and downregulating Vps34 to inhibit autophagosome formation.J Neuroinflammation. 2020 Jan 11;17(1):18. doi: 10.1186/s12974-019-1644-8. J Neuroinflammation. 2020. PMID: 31926553 Free PMC article.
-
Exploring the Use of Dimethyl Fumarate as Microglia Modulator for Neurodegenerative Diseases Treatment.Antioxidants (Basel). 2020 Aug 3;9(8):700. doi: 10.3390/antiox9080700. Antioxidants (Basel). 2020. PMID: 32756501 Free PMC article. Review.
-
Dimethyl Fumarate as Potential Treatment for Alzheimer's Disease: Rationale and Clinical Trial Design.Biomedicines. 2023 May 8;11(5):1387. doi: 10.3390/biomedicines11051387. Biomedicines. 2023. PMID: 37239057 Free PMC article. Review.
Cited by
-
Neuroprotection by Nrf2 via modulating microglial phenotype and phagocytosis after intracerebral hemorrhage.Heliyon. 2023 Feb 16;9(2):e13777. doi: 10.1016/j.heliyon.2023.e13777. eCollection 2023 Feb. Heliyon. 2023. PMID: 36852060 Free PMC article.
-
The Challenge of Dimethyl Fumarate Repurposing in Eye Pathologies.Cells. 2022 Dec 15;11(24):4061. doi: 10.3390/cells11244061. Cells. 2022. PMID: 36552824 Free PMC article. Review.
-
Fluoxetine Potentiates Phagocytosis and Autophagy in Microglia.Front Pharmacol. 2021 Nov 24;12:770610. doi: 10.3389/fphar.2021.770610. eCollection 2021. Front Pharmacol. 2021. PMID: 34899324 Free PMC article.
-
Metabolic regulation of microglial phagocytosis: Implications for Alzheimer's disease therapeutics.Transl Neurodegener. 2023 Oct 31;12(1):48. doi: 10.1186/s40035-023-00382-w. Transl Neurodegener. 2023. PMID: 37908010 Free PMC article. Review.
-
Autophagy in the retinal neurovascular unit: New perspectives into diabetic retinopathy.J Diabetes. 2023 May;15(5):382-396. doi: 10.1111/1753-0407.13373. Epub 2023 Mar 2. J Diabetes. 2023. PMID: 36864557 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

