Electroacupuncture Attenuates Neuropathic Pain and Comorbid Negative Behavior: The Involvement of the Dopamine System in the Amygdala
- PMID: 34025342
- PMCID: PMC8137986
- DOI: 10.3389/fnins.2021.657507
Electroacupuncture Attenuates Neuropathic Pain and Comorbid Negative Behavior: The Involvement of the Dopamine System in the Amygdala
Abstract
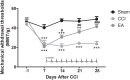
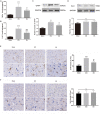
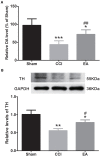
Neuropathic pain (NeuP) is an important clinical problem accompanying negative mood symptoms. Neuroinflammation in the amygdala is critically involved in NeuP, and the dopamine (DA) system acts as an important endogenous anti-inflammatory pathway. Electroacupuncture (EA) can improve the clinical outcomes in NeuP, but the underlying mechanisms have not been fully elucidated. This study was designed to assess the effectiveness of EA on pain and pain-related depressive-like and anxiety-like behaviors and explore the role of the DA system in the effects of EA. Male Sprague-Dawley rats were subjected to the chronic constrictive injury (CCI) model to induce NeuP. EA treatment was carried out for 30 min once every other day for 3 weeks. The results showed that CCI caused mechanical hyperalgesia and depressive and anxiety-like behaviors in rats and neuroinflammation in the amygdala, such as an increased protein level of TNFα and IL-1β and activation of astrocytes. EA treatment significantly improved mechanical allodynia and the emotional dysfunction induced by CCI. The effects of EA were accompanied by markedly decreased expression of TNFα, IL-1β, and glial fibrillary acid protein (GFAP) in the amygdala. Moreover, EA treatment reversed CCI-induced down-regulation of DA concentration, tyrosine hydroxylase (TH) expression, and DRD1 and DRD2 receptors. These results suggest that EA-ameliorated NeuP may possibly be associated with the DA system to inhibit the neuroinflammation in the amygdala.
Keywords: amygdala; dopamine system; electroacupuncture; negative emotion; neuropathic pain.
Copyright © 2021 Zhang, Feng, Pei, Zhang, Chen, Wei, Zhou, Yong and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
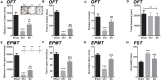
Similar articles
-
Electroacupuncture Alleviates Anxiety-Like Behaviors Induced by Chronic Neuropathic Pain via Regulating Different Dopamine Receptors of the Basolateral Amygdala.Mol Neurobiol. 2022 Sep;59(9):5299-5311. doi: 10.1007/s12035-022-02911-6. Epub 2022 Jun 13. Mol Neurobiol. 2022. PMID: 35696012 Free PMC article.
-
Repeated electroacupuncture treatment attenuated hyperalgesia through suppression of spinal glial activation in chronic neuropathic pain rats.BMC Complement Altern Med. 2018 Feb 21;18(1):74. doi: 10.1186/s12906-018-2134-8. BMC Complement Altern Med. 2018. PMID: 29466978 Free PMC article.
-
[Effect of electroacupuncture on the expression of spinal glial fibrillary acidic protein, tumor necrosis factor-alpha and interleukin-1beta in chronic neuropathic pain rats].Zhen Ci Yan Jiu. 2010 Feb;35(1):12-6. Zhen Ci Yan Jiu. 2010. PMID: 20458900 Chinese.
-
[Effect of electroacupuncture intervention on expression of pain sensory and affection processing related corticotropin-releasing factor receptor mRNA, etc. in the amygdala in neuropathic pain and negative affection rats].Zhen Ci Yan Jiu. 2014 Dec;39(6):448-55. Zhen Ci Yan Jiu. 2014. PMID: 25632568 Chinese.
-
Neuropathic pain in Mali: The current situation, comprehensive hypothesis, which therapeutic strategy for Africa?eNeurologicalSci. 2021 Jan 9;22:100312. doi: 10.1016/j.ensci.2021.100312. eCollection 2021 Mar. eNeurologicalSci. 2021. PMID: 33537467 Free PMC article. Review.
Cited by
-
Research Trends of Acupuncture Therapy for Chronic Pain-Related Depression or Anxiety from 2003 to 2023: A Bibliometric Analysis.J Pain Res. 2023 Dec 15;16:4301-4315. doi: 10.2147/JPR.S436434. eCollection 2023. J Pain Res. 2023. PMID: 38116394 Free PMC article.
-
Hotspots and Trends in Research on Treating Pain with Electroacupuncture: A Bibliometric and Visualization Analysis from 1994 to 2022.J Pain Res. 2023 Nov 3;16:3673-3691. doi: 10.2147/JPR.S422614. eCollection 2023. J Pain Res. 2023. PMID: 37942222 Free PMC article.
-
The analgesic effect of acupuncture in neuropathic pain: regulatory mechanisms of DNA methylation in the brain.Pain Rep. 2024 Oct 23;9(6):e1200. doi: 10.1097/PR9.0000000000001200. eCollection 2024 Dec. Pain Rep. 2024. PMID: 39450409 Free PMC article. Review.
-
Resting-state functional magnetic resonance imaging reveals brain remodeling after Tuina therapy in neuropathic pain model.Front Mol Neurosci. 2023 Jul 12;16:1231374. doi: 10.3389/fnmol.2023.1231374. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37501727 Free PMC article.
-
Electroacupuncture Ameliorates Chronic Inflammatory Pain-Related Anxiety by Activating PV Interneurons in the Anterior Cingulate Cortex.Front Neurosci. 2021 Jul 5;15:691931. doi: 10.3389/fnins.2021.691931. eCollection 2021. Front Neurosci. 2021. PMID: 34290586 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

