Metabolic Plasticity of Neutrophils: Relevance to Pathogen Responses and Cancer
- PMID: 34023325
- PMCID: PMC9270875
- DOI: 10.1016/j.trecan.2021.04.007
Metabolic Plasticity of Neutrophils: Relevance to Pathogen Responses and Cancer
Abstract
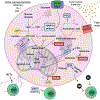
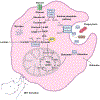
Neutrophils, the most abundant leukocyte population in humans, constantly patrol the body for foreign cells, including pathogens and cancer cells. Once neutrophils are activated, they engage distinct metabolic pathways to fulfill their specialized antipathogen functions. In this review, we examine current research on the metabolism of neutrophil differentiation and antipathogen responses. We also discuss how tumor-associated neutrophils (TANs) can be educated by cytokines and by the nutrient-restrictive milieu of the tumor microenvironment (TME) to suppress antitumor immunity, promote cancer progression, and contribute to biological heterogeneity among tumors. Last, we discuss the clinical implications of circulating neutrophils and infiltrating TANs and consider how targeting TAN metabolism may synergize with cancer immunotherapy.
Keywords: immunotherapy; metabolism; metastasis; neutrophils; tumor microenvironment.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests R.J.D. is a scientific advisor for Agios Pharmaceuticals and Vida Ventures. T.JR has no competing interests to declare.
Figures

Similar articles
-
Neutrophil Heterogeneity in Cancer: From Biology to Therapies.Front Immunol. 2019 Sep 20;10:2155. doi: 10.3389/fimmu.2019.02155. eCollection 2019. Front Immunol. 2019. PMID: 31616408 Free PMC article. Review.
-
Neutrophils in Tumorigenesis: Missing Targets for Successful Next Generation Cancer Therapies?Int J Mol Sci. 2021 Jun 23;22(13):6744. doi: 10.3390/ijms22136744. Int J Mol Sci. 2021. PMID: 34201758 Free PMC article. Review.
-
Neutrophil diversity and plasticity in tumour progression and therapy.Nat Rev Cancer. 2020 Sep;20(9):485-503. doi: 10.1038/s41568-020-0281-y. Epub 2020 Jul 21. Nat Rev Cancer. 2020. PMID: 32694624 Review.
-
Neutrophil: A New Player in Metastatic Cancers.Front Immunol. 2020 Sep 24;11:565165. doi: 10.3389/fimmu.2020.565165. eCollection 2020. Front Immunol. 2020. PMID: 33101283 Free PMC article. Review.
-
CD147‑mediated reprogrammed glycolytic metabolism potentially induces immune escape in the tumor microenvironment (Review).Oncol Rep. 2019 May;41(5):2945-2956. doi: 10.3892/or.2019.7041. Epub 2019 Mar 4. Oncol Rep. 2019. PMID: 30864716 Review.
Cited by
-
Fatty acid metabolism in neutrophils promotes lung damage and bacterial replication during tuberculosis.PLoS Pathog. 2024 Oct 4;20(10):e1012188. doi: 10.1371/journal.ppat.1012188. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39365825 Free PMC article.
-
Neutrophil Extracellular Traps in Tumors and Potential Use of Traditional Herbal Medicine Formulations for Its Regulation.Int J Nanomedicine. 2024 Mar 19;19:2851-2877. doi: 10.2147/IJN.S449181. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38529365 Free PMC article. Review.
-
ZNF8 Orchestrates with Smad3 to Promote Lung Metastasis by Recruiting SMYD3 in Breast Cancer.Adv Sci (Weinh). 2024 Oct;11(40):e2404904. doi: 10.1002/advs.202404904. Epub 2024 Sep 3. Adv Sci (Weinh). 2024. PMID: 39225541 Free PMC article.
-
The systemic-level repercussions of cancer-associated inflammation mediators produced in the tumor microenvironment.Front Endocrinol (Lausanne). 2022 Aug 22;13:929572. doi: 10.3389/fendo.2022.929572. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36072935 Free PMC article. Review.
-
Neutrophil Extracellular Traps (NETs) in Cancer Metastasis.Cancers (Basel). 2021 Dec 6;13(23):6131. doi: 10.3390/cancers13236131. Cancers (Basel). 2021. PMID: 34885240 Free PMC article. Review.
References
-
- Borregaard N (2010) Neutrophils, from marrow to microbes. Immunity 33, 657–670 - PubMed
-
- Hidalgo A et al. (2019) The neutrophil life cycle. Trends Immunol 40, 584–597 - PubMed
-
- Yvan-Charvet L and Ng LG (2019) Granulopoiesis and neutrophil homeostasis: a metabolic, daily balancing act. Trends Immunol. 40, 598–612 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

