Fab-dimerized glycan-reactive antibodies are a structural category of natural antibodies
- PMID: 34019795
- PMCID: PMC8135257
- DOI: 10.1016/j.cell.2021.04.042
Fab-dimerized glycan-reactive antibodies are a structural category of natural antibodies
Abstract
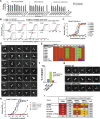
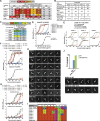
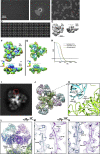
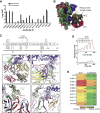
Natural antibodies (Abs) can target host glycans on the surface of pathogens. We studied the evolution of glycan-reactive B cells of rhesus macaques and humans using glycosylated HIV-1 envelope (Env) as a model antigen. 2G12 is a broadly neutralizing Ab (bnAb) that targets a conserved glycan patch on Env of geographically diverse HIV-1 strains using a unique heavy-chain (VH) domain-swapped architecture that results in fragment antigen-binding (Fab) dimerization. Here, we describe HIV-1 Env Fab-dimerized glycan (FDG)-reactive bnAbs without VH-swapped domains from simian-human immunodeficiency virus (SHIV)-infected macaques. FDG Abs also recognized cell-surface glycans on diverse pathogens, including yeast and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike. FDG precursors were expanded by glycan-bearing immunogens in macaques and were abundant in HIV-1-naive humans. Moreover, FDG precursors were predominately mutated IgM+IgD+CD27+, thus suggesting that they originated from a pool of antigen-experienced IgM+ or marginal zone B cells.
Keywords: FDG Abs; Fab dimerization; HIV-1 Env glycans; IgM-memory B cells; SARS-CoV-2 spike glycans; glycan-dependent Ab binding; marginal zone B cells; natural Abs.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests B.A. and W.E.W. are co-founders of Chemitope Technologies, and G.M.L. is a founder of Avidea Technologies that now commercially produce peptides used in our HIV-1 vaccination regimen. The remaining authors declare no competing interests.
Figures










Similar articles
-
Integrity of Glycosylation Processing of a Glycan-Depleted Trimeric HIV-1 Immunogen Targeting Key B-Cell Lineages.J Proteome Res. 2018 Mar 2;17(3):987-999. doi: 10.1021/acs.jproteome.7b00639. Epub 2018 Feb 8. J Proteome Res. 2018. PMID: 29420040 Free PMC article.
-
Diverse recombinant HIV-1 Envs fail to activate B cells expressing the germline B cell receptors of the broadly neutralizing anti-HIV-1 antibodies PG9 and 447-52D.J Virol. 2014 Mar;88(5):2645-57. doi: 10.1128/JVI.03228-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352455 Free PMC article.
-
Vaccine Elicitation of High Mannose-Dependent Neutralizing Antibodies against the V3-Glycan Broadly Neutralizing Epitope in Nonhuman Primates.Cell Rep. 2017 Feb 28;18(9):2175-2188. doi: 10.1016/j.celrep.2017.02.003. Cell Rep. 2017. PMID: 28249163 Free PMC article.
-
Antibody responses to the HIV-1 envelope high mannose patch.Adv Immunol. 2019;143:11-73. doi: 10.1016/bs.ai.2019.08.002. Epub 2019 Sep 11. Adv Immunol. 2019. PMID: 31607367 Free PMC article. Review.
-
Beyond glycan barriers: non-cognate ligands and protein mimicry approaches to elicit broadly neutralizing antibodies for HIV-1.J Biomed Sci. 2024 Aug 21;31(1):83. doi: 10.1186/s12929-024-01073-y. J Biomed Sci. 2024. PMID: 39169357 Free PMC article. Review.
Cited by
-
Antibody interfaces revealed through structural mining.Comput Struct Biotechnol J. 2022 Aug 31;20:4952-4968. doi: 10.1016/j.csbj.2022.08.048. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36147680 Free PMC article.
-
Analysis of two cooperating antibodies unveils immune pressure imposed on HIV Env to elicit a V3-glycan supersite broadly neutralizing antibody lineage.Front Immunol. 2022 Sep 26;13:962939. doi: 10.3389/fimmu.2022.962939. eCollection 2022. Front Immunol. 2022. PMID: 36225920 Free PMC article.
-
Single-molecule imaging reveals allosteric stimulation of SARS-CoV-2 spike receptor binding domain by host sialic acid.Sci Adv. 2024 Jul 19;10(29):eadk4920. doi: 10.1126/sciadv.adk4920. Epub 2024 Jul 17. Sci Adv. 2024. PMID: 39018397 Free PMC article.
-
Neonatal SHIV infection in rhesus macaques elicited heterologous HIV-1-neutralizing antibodies.Cell Rep. 2023 Mar 28;42(3):112255. doi: 10.1016/j.celrep.2023.112255. Epub 2023 Mar 14. Cell Rep. 2023. PMID: 36924501 Free PMC article.
-
Strategies for induction of HIV-1 envelope-reactive broadly neutralizing antibodies.J Int AIDS Soc. 2021 Nov;24 Suppl 7(Suppl 7):e25831. doi: 10.1002/jia2.25831. J Int AIDS Soc. 2021. PMID: 34806332 Free PMC article. Review.
References
-
- Alexander J., del Guercio M.F., Maewal A., Qiao L., Fikes J., Chesnut R.W., Paulson J., Bundle D.R., DeFrees S., Sette A. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J. Immunol. 2000;164:1625–1633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI100645/AI/NIAID NIH HHS/United States
- T32 GM136577/GM/NIGMS NIH HHS/United States
- HHSN272201800004C/AI/NIAID NIH HHS/United States
- UC6 AI058607/AI/NIAID NIH HHS/United States
- R01 AI140897/AI/NIAID NIH HHS/United States
- P01 AI131251/AI/NIAID NIH HHS/United States
- R01 AI165080/AI/NIAID NIH HHS/United States
- U24 GM129539/GM/NIGMS NIH HHS/United States
- R01 AI120801/AI/NIAID NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 AI131331/AI/NIAID NIH HHS/United States
- R01 AI160607/AI/NIAID NIH HHS/United States
- R01 AI145687/AI/NIAID NIH HHS/United States
- R01 AI128832/AI/NIAID NIH HHS/United States
- T32 GM007171/GM/NIGMS NIH HHS/United States
- P30 AI064518/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

