Live-cell imaging of glucose-induced metabolic coupling of β and α cell metabolism in health and type 2 diabetes
- PMID: 34012065
- PMCID: PMC8134470
- DOI: 10.1038/s42003-021-02113-1
Live-cell imaging of glucose-induced metabolic coupling of β and α cell metabolism in health and type 2 diabetes
Abstract
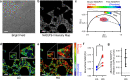
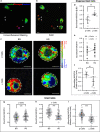
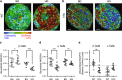
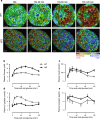
Type 2 diabetes is characterized by β and α cell dysfunction. We used phasor-FLIM (Fluorescence Lifetime Imaging Microscopy) to monitor oxidative phosphorylation and glycolysis in living islet cells before and after glucose stimulation. In healthy cells, glucose enhanced oxidative phosphorylation in β cells and suppressed oxidative phosphorylation in α cells. In Type 2 diabetes, glucose increased glycolysis in β cells, and only partially suppressed oxidative phosphorylation in α cells. FLIM uncovers key perturbations in glucose induced metabolism in living islet cells and provides a sensitive tool for drug discovery in diabetes.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Activation of Transmembrane Bile Acid Receptor TGR5 Modulates Pancreatic Islet α Cells to Promote Glucose Homeostasis.J Biol Chem. 2016 Mar 25;291(13):6626-40. doi: 10.1074/jbc.M115.699504. Epub 2016 Jan 12. J Biol Chem. 2016. PMID: 26757816 Free PMC article.
-
Pancreatic islet amyloidosis, beta-cell apoptosis, and alpha-cell proliferation are determinants of islet remodeling in type-2 diabetic baboons.Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):13992-7. doi: 10.1073/pnas.0906471106. Epub 2009 Jul 30. Proc Natl Acad Sci U S A. 2009. PMID: 19666551 Free PMC article.
-
Long-term liraglutide treatment is associated with increased insulin content and secretion in β-cells, and a loss of α-cells in ZDF rats.Pharmacol Res. 2013 Oct;76:58-66. doi: 10.1016/j.phrs.2013.07.005. Epub 2013 Jul 26. Pharmacol Res. 2013. PMID: 23891763
-
Improved glucose regulation in type 2 diabetic patients with DPP-4 inhibitors: focus on alpha and beta cell function and lipid metabolism.Diabetologia. 2016 May;59(5):907-17. doi: 10.1007/s00125-016-3899-2. Epub 2016 Feb 19. Diabetologia. 2016. PMID: 26894277 Review.
-
The role of GIP in α-cells and glucagon secretion.Peptides. 2020 Mar;125:170213. doi: 10.1016/j.peptides.2019.170213. Epub 2019 Nov 27. Peptides. 2020. PMID: 31785304 Free PMC article. Review.
Cited by
-
KATP channel activity and slow oscillations in pancreatic beta cells are regulated by mitochondrial ATP production.J Physiol. 2023 Dec;601(24):5655-5667. doi: 10.1113/JP284982. Epub 2023 Nov 20. J Physiol. 2023. PMID: 37983196 Free PMC article.
-
Subcellular Feature-Based Classification of α and β Cells Using Soft X-ray Tomography.Cells. 2024 May 18;13(10):869. doi: 10.3390/cells13100869. Cells. 2024. PMID: 38786091 Free PMC article.
-
HumanIslets: An integrated platform for human islet data access and analysis.bioRxiv [Preprint]. 2024 Jun 22:2024.06.19.599613. doi: 10.1101/2024.06.19.599613. bioRxiv. 2024. PMID: 38948734 Free PMC article. Preprint.
-
More than double the fun with two-photon excitation microscopy.Commun Biol. 2024 Mar 26;7(1):364. doi: 10.1038/s42003-024-06057-0. Commun Biol. 2024. PMID: 38531976 Free PMC article. Review.
-
Bayesian metamodeling of complex biological systems across varying representations.Proc Natl Acad Sci U S A. 2021 Aug 31;118(35):e2104559118. doi: 10.1073/pnas.2104559118. Proc Natl Acad Sci U S A. 2021. PMID: 34453000 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

