Cardiomyocyte contractile impairment in heart failure results from reduced BAG3-mediated sarcomeric protein turnover
- PMID: 34011988
- PMCID: PMC8134551
- DOI: 10.1038/s41467-021-23272-z
Cardiomyocyte contractile impairment in heart failure results from reduced BAG3-mediated sarcomeric protein turnover
Abstract
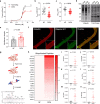
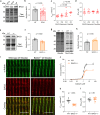
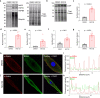
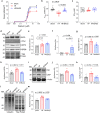
The association between reduced myofilament force-generating capacity (Fmax) and heart failure (HF) is clear, however the underlying molecular mechanisms are poorly understood. Here, we show impaired Fmax arises from reduced BAG3-mediated sarcomere turnover. Myofilament BAG3 expression decreases in human HF and positively correlates with Fmax. We confirm this relationship using BAG3 haploinsufficient mice, which display reduced Fmax and increased myofilament ubiquitination, suggesting impaired protein turnover. We show cardiac BAG3 operates via chaperone-assisted selective autophagy (CASA), conserved from skeletal muscle, and confirm sarcomeric CASA complex localization is BAG3/proteotoxic stress-dependent. Using mass spectrometry, we characterize the myofilament CASA interactome in the human heart and identify eight clients of BAG3-mediated turnover. To determine if increasing BAG3 expression in HF can restore sarcomere proteostasis/Fmax, HF mice were treated with rAAV9-BAG3. Gene therapy fully rescued Fmax and CASA protein turnover after four weeks. Our findings indicate BAG3-mediated sarcomere turnover is fundamental for myofilament functional maintenance.
Conflict of interest statement
A.M.F. has equity in and is a director of Renovacor, Inc., a biotechnology company developing gene therapy for patients with BAG3 genetic variants. The other authors declare no competing interests.
Figures



Similar articles
-
Dysregulated Autophagy and Sarcomere Dysfunction in Patients With Heart Failure With Co-Occurrence of P63A and P380S BAG3 Variants.J Am Heart Assoc. 2023 Dec 19;12(24):e029938. doi: 10.1161/JAHA.123.029938. Epub 2023 Dec 18. J Am Heart Assoc. 2023. PMID: 38108245 Free PMC article.
-
BAG3 expression and sarcomere localization in the human heart are linked to HSF-1 and are differentially affected by sex and disease.Am J Physiol Heart Circ Physiol. 2021 Jun 1;320(6):H2339-H2350. doi: 10.1152/ajpheart.00419.2020. Epub 2021 May 14. Am J Physiol Heart Circ Physiol. 2021. PMID: 33989081 Free PMC article.
-
Transcriptional upregulation of Bag3, a chaperone-assisted selective autophagy factor, in animal models of KY-deficient hereditary myopathy.Dis Model Mech. 2018 Jul 6;11(7):dmm033225. doi: 10.1242/dmm.033225. Dis Model Mech. 2018. PMID: 29914939 Free PMC article.
-
BAG3: a new player in the heart failure paradigm.Heart Fail Rev. 2015 Jul;20(4):423-34. doi: 10.1007/s10741-015-9487-6. Heart Fail Rev. 2015. PMID: 25925243 Free PMC article. Review.
-
Advances in the role and mechanism of BAG3 in dilated cardiomyopathy.Heart Fail Rev. 2021 Jan;26(1):183-194. doi: 10.1007/s10741-019-09899-7. Heart Fail Rev. 2021. PMID: 31808029 Review.
Cited by
-
Molecular effects of cardiac contractility modulation in patients with heart failure of ischemic aetiology uncovered by transcriptome analysis.Front Cardiovasc Med. 2024 Feb 1;11:1321005. doi: 10.3389/fcvm.2024.1321005. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38361583 Free PMC article.
-
BAG Family Members as Mitophagy Regulators in Mammals.Cells. 2022 Feb 15;11(4):681. doi: 10.3390/cells11040681. Cells. 2022. PMID: 35203329 Free PMC article. Review.
-
Going Further With Half as Much: It's in the BAG.JACC Basic Transl Sci. 2023 Sep 25;8(9):1212-1214. doi: 10.1016/j.jacbts.2023.06.008. eCollection 2023 Sep. JACC Basic Transl Sci. 2023. PMID: 37791297 Free PMC article.
-
Simultaneous proteome localization and turnover analysis reveals spatiotemporal features of protein homeostasis disruptions.Nat Commun. 2024 Mar 11;15(1):2207. doi: 10.1038/s41467-024-46600-5. Nat Commun. 2024. PMID: 38467653 Free PMC article.
-
The importance of myosin ubiquitination status for cardiac remodeling: USP20 has entered the chat.Am J Physiol Heart Circ Physiol. 2024 Nov 1;327(5):H1306-H1308. doi: 10.1152/ajpheart.00712.2024. Epub 2024 Oct 25. Am J Physiol Heart Circ Physiol. 2024. PMID: 39453432 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

