Stem cells for the treatment of glioblastoma: a 20-year perspective
- PMID: 34006134
- PMCID: PMC8162173
- DOI: 10.2217/cns-2020-0026
Stem cells for the treatment of glioblastoma: a 20-year perspective
Abstract
Glioblastoma, the deadliest form of primary brain tumor, remains a disease without cure. Treatment resistance is in large part attributed to limitations in the delivery and distribution of therapeutic agents. Over the last 20 years, numerous preclinical studies have demonstrated the feasibility and efficacy of stem cells as antiglioma agents, leading to the development of trials to test these therapies in the clinic. In this review we present and analyze these studies, discuss mechanisms underlying their beneficial effect and highlight experimental progress, limitations and the emergence of promising new therapeutic avenues. We hope to increase awareness of the advantages brought by stem cells for the treatment of glioblastoma and inspire further studies that will lead to accelerated implementation of effective therapies.
Keywords: enzyme/prodrug; exosomes; glioblastoma; immunologic cell death; mesenchymal stem cells; nanoparticles; neural stem cells; oncolytic virotherapy; stem cells; therapeutic stem cells.
Plain language summary
Lay abstract Glioblastoma is the deadliest and most common form of brain tumor, for which there is no cure. It is very difficult to deliver medicine to the tumor cells, because they spread out widely into the normal brain, and local blood vessels represent a barrier that most medicines cannot cross. It was shown, in many studies over the last 20 years, that stem cells are attracted toward the tumor and that they can deliver many kinds of therapeutic agents directly to brain cancer cells and shrink the tumor. In this review we analyze these studies and present new discoveries that can be used to make stem cell therapies for glioblastoma more effective to prolong the life of patients with brain tumors.
Conflict of interest statement
Grant support was provided by NIH/NINDS R21NS107879 (AC). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures
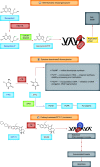
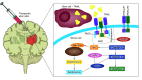

Similar articles
-
Stem cells as delivery vehicles for oncolytic adenoviral virotherapy.Curr Gene Ther. 2009 Oct;9(5):389-95. doi: 10.2174/156652309789753347. Curr Gene Ther. 2009. PMID: 19860653 Free PMC article. Review.
-
Stem cell-based therapies for tumors in the brain: are we there yet?Neuro Oncol. 2016 Aug;18(8):1066-78. doi: 10.1093/neuonc/now096. Epub 2016 Jun 9. Neuro Oncol. 2016. PMID: 27282399 Free PMC article. Review.
-
A preclinical evaluation of neural stem cell-based cell carrier for targeted antiglioma oncolytic virotherapy.J Natl Cancer Inst. 2013 Jul 3;105(13):968-77. doi: 10.1093/jnci/djt141. J Natl Cancer Inst. 2013. PMID: 23821758 Free PMC article.
-
Gene therapeutics: the future of brain tumor therapy?Expert Rev Anticancer Ther. 2006 Jul;6(7):1053-64. doi: 10.1586/14737140.6.7.1053. Expert Rev Anticancer Ther. 2006. PMID: 16831077 Review.
-
Neural Stem Cell-Based Therapies and Glioblastoma Management: Current Evidence and Clinical Challenges.Int J Mol Sci. 2021 Feb 24;22(5):2258. doi: 10.3390/ijms22052258. Int J Mol Sci. 2021. PMID: 33668356 Free PMC article. Review.
Cited by
-
Advanced Cell Therapies for Glioblastoma.Front Immunol. 2022 Aug 16;13:904133. doi: 10.3389/fimmu.2022.904133. eCollection 2022. Front Immunol. 2022. PMID: 36052072 Free PMC article. Review.
-
Mesenchymal stem cell therapy for neurological disorders: The light or the dark side of the force?Front Bioeng Biotechnol. 2023 Feb 28;11:1139359. doi: 10.3389/fbioe.2023.1139359. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36926687 Free PMC article. Review.
-
Challenges and strategies toward oncolytic virotherapy for leptomeningeal metastasis.J Transl Med. 2024 Nov 5;22(1):1000. doi: 10.1186/s12967-024-05794-4. J Transl Med. 2024. PMID: 39501324 Free PMC article. Review.
-
Gene therapy in glioblastoma multiforme: Can it be a role changer?Heliyon. 2024 Feb 24;10(5):e27087. doi: 10.1016/j.heliyon.2024.e27087. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38439834 Free PMC article. Review.
-
Cell-Based Therapy for the Treatment of Glioblastoma: An Update from Preclinical to Clinical Studies.Cells. 2021 Dec 30;11(1):116. doi: 10.3390/cells11010116. Cells. 2021. PMID: 35011678 Free PMC article. Review.
References
-
- His W. Die Entwickelung des menschlichen Gehirns: während der ersten Monate. S. Hirzel; (1904).
-
- Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the CNS. Annu. Rev. Neurosci. 18(1), 159–192 (1995). - PubMed
-
- Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, Van Der Kooy D. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 19(9), 387–393 (1996). - PubMed
-
- Temple S. Division and differentiation of isolated CNS blast cells in microculture. Nature 340(6233), 471–473 (1989). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
