Calibration of cell-intrinsic interleukin-2 response thresholds guides design of a regulatory T cell biased agonist
- PMID: 34003116
- PMCID: PMC8131104
- DOI: 10.7554/eLife.65777
Calibration of cell-intrinsic interleukin-2 response thresholds guides design of a regulatory T cell biased agonist
Abstract
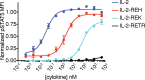
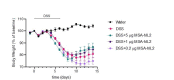
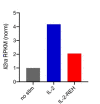
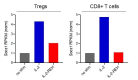
Interleukin-2 is a pleiotropic cytokine that mediates both pro- and anti-inflammatory functions. Immune cells naturally differ in their sensitivity to IL-2 due to cell type and activation state-dependent expression of receptors and signaling pathway components. To probe differences in IL-2 signaling across cell types, we used structure-based design to create and profile a series of IL-2 variants with the capacity to titrate maximum signal strength in fine increments. One of these partial agonists, IL-2-REH, specifically expanded Foxp3+ regulatory T cells with reduced activity on CD8+ T cells due to cell type-intrinsic differences in IL-2 signaling. IL-2-REH elicited cell type-dependent differences in gene expression and provided mixed therapeutic results: showing benefit in the in vivo mouse dextran sulfate sodium (DSS) model of colitis, but no therapeutic efficacy in a transfer colitis model. Our findings show that cytokine partial agonists can be used to calibrate intrinsic differences in response thresholds across responding cell types to narrow pleiotropic actions, which may be generalizable to other cytokine and growth factor systems.
Keywords: IL-2; biochemistry; chemical biology; cytokine signaling; human; immunology; inflammation; mouse.
Conflict of interest statement
CG, LS, SM Coauthor on a patent which includes discoveries described in this manuscript (WO/2019/104092). HW, FM, PL, MP, PH, IK, NN, TH, LP, MK, PB, LS, EM, JP, WL No competing interests declared, KG Coauthor on a patent which includes discoveries described in this manuscript (WO/2019/104092). Founder of Synthekine.
Figures




Similar articles
-
Estrogen receptor β activation ameliorates DSS-induced chronic colitis by inhibiting inflammation and promoting Treg differentiation.Int Immunopharmacol. 2019 Dec;77:105971. doi: 10.1016/j.intimp.2019.105971. Epub 2019 Oct 31. Int Immunopharmacol. 2019. PMID: 31678865
-
Interleukin-36β exacerbates DSS-induce acute colitis via inhibiting Foxp3+ regulatory T cell response and increasing Th2 cell response.Int Immunopharmacol. 2022 Jul;108:108762. doi: 10.1016/j.intimp.2022.108762. Epub 2022 Apr 15. Int Immunopharmacol. 2022. PMID: 35436743
-
Yogurt starter cultures of Streptococcus thermophilus and Lactobacillus bulgaricus ameliorate symptoms and modulate the immune response in a mouse model of dextran sulfate sodium-induced colitis.J Dairy Sci. 2019 Jan;102(1):37-53. doi: 10.3168/jds.2018-14520. Epub 2018 Oct 19. J Dairy Sci. 2019. PMID: 30343915
-
IL-33 Drives Expansion of Type 2 Innate Lymphoid Cells and Regulatory T Cells and Protects Mice From Severe, Acute Colitis.Front Immunol. 2021 Jul 15;12:669787. doi: 10.3389/fimmu.2021.669787. eCollection 2021. Front Immunol. 2021. PMID: 34335571 Free PMC article.
-
Strategies to therapeutically modulate cytokine action.Nat Rev Drug Discov. 2023 Oct;22(10):827-854. doi: 10.1038/s41573-023-00746-x. Epub 2023 Aug 4. Nat Rev Drug Discov. 2023. PMID: 37542128 Review.
Cited by
-
Regulatory T cells in dominant immunologic tolerance.J Allergy Clin Immunol. 2024 Jan;153(1):28-41. doi: 10.1016/j.jaci.2023.09.025. Epub 2023 Sep 29. J Allergy Clin Immunol. 2024. PMID: 37778472 Free PMC article. Review.
-
The Expression of Selected Cytokine Genes in the Livers of Young Castrated Bucks after Supplementation with a Mixture of Dry Curcuma longa and Rosmarinus officinalis Extracts.Animals (Basel). 2023 Nov 12;13(22):3489. doi: 10.3390/ani13223489. Animals (Basel). 2023. PMID: 38003107 Free PMC article.
-
Facile discovery of surrogate cytokine agonists.Cell. 2022 Apr 14;185(8):1414-1430.e19. doi: 10.1016/j.cell.2022.02.025. Epub 2022 Mar 23. Cell. 2022. PMID: 35325595 Free PMC article.
-
Emerging principles of cytokine pharmacology and therapeutics.Nat Rev Drug Discov. 2023 Jan;22(1):21-37. doi: 10.1038/s41573-022-00557-6. Epub 2022 Sep 21. Nat Rev Drug Discov. 2023. PMID: 36131080 Free PMC article. Review.
-
Molecular Engineering of Interleukin-2 for Enhanced Therapeutic Activity in Autoimmune Diseases.BioDrugs. 2024 Mar;38(2):227-248. doi: 10.1007/s40259-023-00635-0. Epub 2023 Nov 24. BioDrugs. 2024. PMID: 37999893 Free PMC article. Review.
References
-
- Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. Journal of Experimental Medicine. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

