A Novel Series of Indole Alkaloid Derivatives Inhibit Dengue and Zika Virus Infection by Interference with the Viral Replication Complex
- PMID: 34001508
- PMCID: PMC8284442
- DOI: 10.1128/AAC.02349-20
A Novel Series of Indole Alkaloid Derivatives Inhibit Dengue and Zika Virus Infection by Interference with the Viral Replication Complex
Abstract
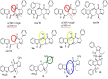
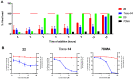
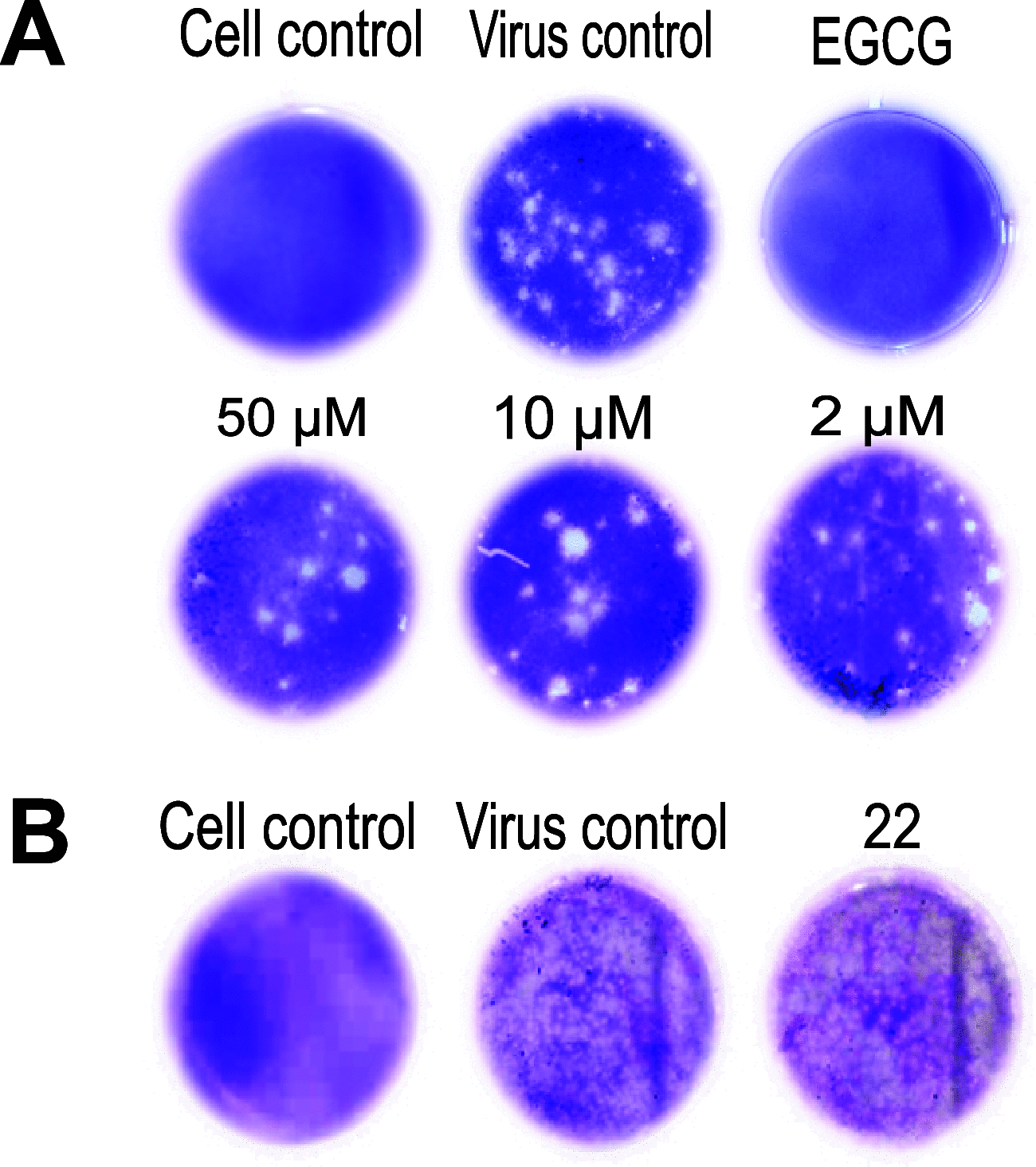
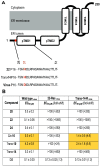
Here, we identified a novel class of compounds which demonstrated good antiviral activity against dengue and Zika virus infection. These derivatives constitute intermediates in the synthesis of indole (ervatamine-silicine) alkaloids and share a tetracyclic structure, with an indole and a piperidine fused to a seven-membered carbocyclic ring. Structure-activity relationship studies indicated the importance of substituent at position C-6 and especially the presence of a benzyl ester for the activity and cytotoxicity of the molecules. In addition, the stereochemistry at C-7 and C-8, as well as the presence of an oxazolidine ring, influenced the potency of the compounds. Mechanism of action studies with two analogues of this family (compounds 22 and trans-14) showed that this class of molecules can suppress viral infection during the later stages of the replication cycle (RNA replication/assembly). Moreover, a cell-dependent antiviral profile of the compounds against several Zika strains was observed, possibly implying the involvement of a cellular factor(s) in the activity of the molecules. Sequencing of compound-resistant Zika mutants revealed a single nonsynonymous amino acid mutation (aspartic acid to histidine) at the beginning of the predicted transmembrane domain 1 of NS4B protein, which plays a vital role in the formation of the viral replication complex. To conclude, our study provides detailed information on a new class of NS4B-associated inhibitors and strengthens the importance of identifying host-virus interactions in order to tackle flavivirus infections.
Keywords: NS4B protein; SAR studies; Zika virus; cross resistance; dengue virus; indole alkaloids; mechanisms of action.
Figures

Similar articles
-
Amaryllidaceae Alkaloid Cherylline Inhibits the Replication of Dengue and Zika Viruses.Antimicrob Agents Chemother. 2021 Aug 17;65(9):e0039821. doi: 10.1128/AAC.00398-21. Epub 2021 Aug 17. Antimicrob Agents Chemother. 2021. PMID: 34152811 Free PMC article.
-
Indole alkaloids inhibit zika and chikungunya virus infection in different cell lines.BMC Complement Med Ther. 2021 Aug 28;21(1):216. doi: 10.1186/s12906-021-03386-z. BMC Complement Med Ther. 2021. PMID: 34454481 Free PMC article.
-
RNA Helicase A Is an Important Host Factor Involved in Dengue Virus Replication.J Virol. 2019 Feb 5;93(4):e01306-18. doi: 10.1128/JVI.01306-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463971 Free PMC article.
-
Flavivirus nonstructural proteins and replication complexes as antiviral drug targets.Curr Opin Virol. 2023 Apr;59:101305. doi: 10.1016/j.coviro.2023.101305. Epub 2023 Mar 2. Curr Opin Virol. 2023. PMID: 36870091 Free PMC article. Review.
-
The Dengue Virus Replication Complex: From RNA Replication to Protein-Protein Interactions to Evasion of Innate Immunity.Adv Exp Med Biol. 2018;1062:115-129. doi: 10.1007/978-981-10-8727-1_9. Adv Exp Med Biol. 2018. PMID: 29845529 Review.
Cited by
-
Dengue virus NS4B protein as a target for developing antivirals.Front Cell Infect Microbiol. 2022 Aug 9;12:959727. doi: 10.3389/fcimb.2022.959727. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36017362 Free PMC article. Review.
-
Bisbenzylisoquinoline Alkaloids of Cissampelos Sympodialis With in Vitro Antiviral Activity Against Zika Virus.Front Pharmacol. 2021 Sep 15;12:743541. doi: 10.3389/fphar.2021.743541. eCollection 2021. Front Pharmacol. 2021. PMID: 34603056 Free PMC article.
-
Computational Identification of Potential Multitarget Inhibitors of Nipah Virus by Molecular Docking and Molecular Dynamics.Microorganisms. 2022 Jun 9;10(6):1181. doi: 10.3390/microorganisms10061181. Microorganisms. 2022. PMID: 35744699 Free PMC article.
-
Identification of Polyphenol Derivatives as Novel SARS-CoV-2 and DENV Non-Nucleoside RdRp Inhibitors.Molecules. 2022 Dec 25;28(1):160. doi: 10.3390/molecules28010160. Molecules. 2022. PMID: 36615354 Free PMC article.
-
Alkaloids as potential antivirals. A comprehensive review.Nat Prod Bioprospect. 2023 Jan 4;13(1):4. doi: 10.1007/s13659-022-00366-9. Nat Prod Bioprospect. 2023. PMID: 36598588 Free PMC article. Review.
References
-
- Bollati M, Alvarez K, Assenberg R, Baronti C, Canard B, Cook S, Coutard B, Decroly E, de Lamballerie X, Gould EA, Grard G, Grimes JM, Hilgenfeld R, Jansson AM, Malet H, Mancini EJ, Mastrangelo E, Mattevi A, Milani M, Moureau G, Neyts J, Owens RJ, Ren J, Selisko B, Speroni S, Steuber H, Stuart DI, Unge T, Bolognesi M. 2010. Structure and functionality in flavivirus NS-proteins: perspectives for drug design. Antiviral Res 87:125–148. 10.1016/j.antiviral.2009.11.009. - DOI - PMC - PubMed
-
- Rezende HR, Romano CM, Claro IM, Caleiro GS, Sabino EC, Felix AC, Bissoli J, Hill J, Faria NR, da Silva TCC, Brioschi Santos AP, Junior CC, Vicente CR. 2020. First report of Aedes albopictus infected by dengue and Zika virus in a rural outbreak in Brazil. PLoS One 15:e0229847. 10.1371/journal.pone.0229847. - DOI - PMC - PubMed
-
- Blohm GM, Lednicky JA, Márquez M, White SK, Loeb JC, Pacheco CA, Nolan DJ, Paisie T, Salemi M, Rodriguez-Morales AJ, Morris JG, Jr, Pulliam JRC, Paniz-Mondolfi AE. 2018. Evidence for mother-to-child transmission of Zika virus through breast milk. Clin Infect Dis 66:1120–1121. 10.1093/cid/cix968. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

