Decay of Fc-dependent antibody functions after mild to moderate COVID-19
- PMID: 33997824
- PMCID: PMC8106889
- DOI: 10.1016/j.xcrm.2021.100296
Decay of Fc-dependent antibody functions after mild to moderate COVID-19
Abstract
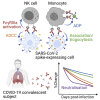
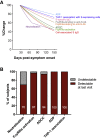
The capacity of antibodies to engage with immune cells via the Fc region is important in preventing and controlling many infectious diseases. The evolution of such antibodies during convalescence from coronavirus disease 2019 (COVID-19) is largely unknown. We develop assays to measure Fc-dependent antibody functions against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S)-expressing cells in serial samples from subjects primarily with mild-moderate COVID-19 up to 149 days post-infection. We find that S-specific antibodies capable of engaging Fcγ receptors decay over time, with S-specific antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent phagocytosis (ADP) activity within plasma declining accordingly. Although there is significant decay in ADCC and ADP activity, they remain readily detectable in almost all subjects at the last time point studied (94%) in contrast with neutralization activity (70%). Although it remains unclear the degree to which Fc effector functions contribute to protection against SARS-CoV-2 re-infection, our results indicate that antibodies with Fc effector functions persist longer than neutralizing antibodies.
Keywords: ADCC; ADP; COVID-19; Fc effector functions; SARS-CoV-2; antibody; convalescence; decay; phagocytosis; trogocytosis.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
COVID-19 Severity Is Associated with Differential Antibody Fc-Mediated Innate Immune Functions.mBio. 2021 Apr 20;12(2):e00281-21. doi: 10.1128/mBio.00281-21. mBio. 2021. PMID: 33879594 Free PMC article.
-
Deciphering Fc-effector functions against SARS-CoV-2.Trends Microbiol. 2024 Aug;32(8):756-768. doi: 10.1016/j.tim.2024.01.005. Epub 2024 Feb 15. Trends Microbiol. 2024. PMID: 38365562 Review.
-
Anti-Severe Acute Respiratory Syndrome Coronavirus 2 Hyperimmune Immunoglobulin Demonstrates Potent Neutralization and Antibody-Dependent Cellular Cytotoxicity and Phagocytosis Through N and S Proteins.J Infect Dis. 2022 Mar 15;225(6):938-946. doi: 10.1093/infdis/jiab540. J Infect Dis. 2022. PMID: 34693968 Free PMC article.
-
A Fc-enhanced NTD-binding non-neutralizing antibody delays virus spread and synergizes with a nAb to protect mice from lethal SARS-CoV-2 infection.Cell Rep. 2022 Feb 15;38(7):110368. doi: 10.1016/j.celrep.2022.110368. Epub 2022 Jan 25. Cell Rep. 2022. PMID: 35123652 Free PMC article.
-
Protective non-neutralizing SARS-CoV-2 monoclonal antibodies.Trends Immunol. 2024 Aug;45(8):609-624. doi: 10.1016/j.it.2024.06.003. Epub 2024 Jul 20. Trends Immunol. 2024. PMID: 39034185 Review.
Cited by
-
The humoral response and antibodies against SARS-CoV-2 infection.Nat Immunol. 2022 Jul;23(7):1008-1020. doi: 10.1038/s41590-022-01248-5. Epub 2022 Jun 27. Nat Immunol. 2022. PMID: 35761083 Review.
-
Despite delayed kinetics, people living with HIV achieve equivalent antibody function after SARS-CoV-2 infection or vaccination.Front Immunol. 2023 Aug 3;14:1231276. doi: 10.3389/fimmu.2023.1231276. eCollection 2023. Front Immunol. 2023. PMID: 37600825 Free PMC article.
-
T follicular helper cells in the humoral immune response to SARS-CoV-2 infection and vaccination.J Leukoc Biol. 2022 Feb;111(2):355-365. doi: 10.1002/JLB.5MR0821-464R. Epub 2021 Nov 3. J Leukoc Biol. 2022. PMID: 34730247 Free PMC article. Review.
-
BNT162b2 induced neutralizing and non-neutralizing antibody functions against SARSCoV-2 diminish with age.medRxiv [Preprint]. 2022 Aug 16:2022.08.12.22278726. doi: 10.1101/2022.08.12.22278726. medRxiv. 2022. Update in: Cell Rep. 2022 Oct 25;41(4):111544. doi: 10.1016/j.celrep.2022.111544 PMID: 36032979 Free PMC article. Updated. Preprint.
-
Complement activation induces excessive T cell cytotoxicity in severe COVID-19.Cell. 2022 Feb 3;185(3):493-512.e25. doi: 10.1016/j.cell.2021.12.040. Epub 2021 Dec 28. Cell. 2022. PMID: 35032429 Free PMC article.
References
-
- Juno J.A., Tan H.X., Lee W.S., Reynaldi A., Kelly H.G., Wragg K., Esterbauer R., Kent H.E., Batten C.J., Mordant F.L. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat. Med. 2020;26:1428–1434. - PubMed
-
- Piccoli L., Park Y.J., Tortorici M.A., Czudnochowski N., Walls A.C., Beltramello M., Silacci-Fregni C., Pinto D., Rosen L.E., Bowen J.E. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 Spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020;183:1024–1042.e21. - PMC - PubMed
-
- Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q., Luo Y., Chan J.F., Sahi V., Figueroa A. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

