Na,K-ATPase as a target for endogenous cardiotonic steroids: What's the evidence?
- PMID: 33997173
- PMCID: PMC8093582
- DOI: 10.1016/j.gendis.2020.01.008
Na,K-ATPase as a target for endogenous cardiotonic steroids: What's the evidence?
Abstract
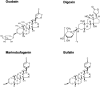
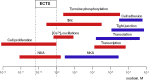
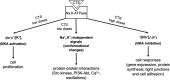
With an exception of few reports, the plasma concentration of ouabain and marinobufagenin, mostly studied cardiotonic steroids (CTS) assessed by immunoassay techniques, is less than 1 nM. During the last 3 decades, the implication of these endogenous CTS in the pathogenesis of hypertension and other volume-expanded disorders is widely disputed. The threshold for inhibition by CTS of human and rodent α1-Na,K-ATPase is ∼1 and 1000 nM, respectively, that rules out the functioning of endogenous CTS (ECTS) as natriuretic hormones and regulators of cell adhesion, cell-to-cell communication, gene transcription and translation, which are mediated by dissipation of the transmembrane gradients of monovalent cations. In several types of cells ouabain and marinobufagenin at concentrations corresponding to its plasma level activate Na,K-ATPase, decrease the [Na+]i/[K+]i-ratio and increase cell proliferation. Possible physiological significance and mechanism of non-canonical Na+ i/K+ i-dependent and Na+ i/K+ i-independent cell responses to CTS are discussed.
Keywords: Cell proliferation; Cellular signaling; Endogenous cardiotonic steroids; Na+,K+-ATPase; Transcription; Translation.
© 2020 Chongqing Medical University. Production and hosting by Elsevier B.V.
Figures
Similar articles
-
The Na(+)/K(+)-ATPase as [K(+)](o) sensor: Role in cardiovascular disease pathogenesis and augmented production of endogenous cardiotonic steroids.Pathophysiology. 2006 Dec;13(4):209-16. doi: 10.1016/j.pathophys.2006.06.001. Epub 2006 Jul 20. Pathophysiology. 2006. PMID: 16857351
-
Binding of ouabain and marinobufagenin leads to different structural changes in Na,K-ATPase and depends on the enzyme conformation.FEBS Lett. 2015 Sep 14;589(19 Pt B):2668-74. doi: 10.1016/j.febslet.2015.08.011. Epub 2015 Aug 20. FEBS Lett. 2015. PMID: 26297827
-
Comparative Action of Cardiotonic Steroids on Intracellular Processes in Rat Cortical Neurons.Biochemistry (Mosc). 2018 Feb;83(2):140-151. doi: 10.1134/S0006297918020062. Biochemistry (Mosc). 2018. PMID: 29618300
-
Na⁺i,K⁺i-Dependent and -Independent Signaling Triggered by Cardiotonic Steroids: Facts and Artifacts.Molecules. 2017 Apr 14;22(4):635. doi: 10.3390/molecules22040635. Molecules. 2017. PMID: 28420099 Free PMC article. Review.
-
Ouabain-Induced Cell Death and Survival. Role of α1-Na,K-ATPase-Mediated Signaling and [Na+]i/[K+]i-Dependent Gene Expression.Front Physiol. 2020 Sep 4;11:1060. doi: 10.3389/fphys.2020.01060. eCollection 2020. Front Physiol. 2020. PMID: 33013454 Free PMC article. Review.
Cited by
-
Endogenous bufadienolides, mineralocorticoid receptor antagonists and fibrosis in chronic kidney disease.Front Pharmacol. 2024 Sep 4;15:1431216. doi: 10.3389/fphar.2024.1431216. eCollection 2024. Front Pharmacol. 2024. PMID: 39295945 Free PMC article. Review.
-
Mesenchymal Stem/Stromal Cells in Three-Dimensional Cell Culture: Ion Homeostasis and Ouabain-Induced Apoptosis.Biomedicines. 2023 Jan 21;11(2):301. doi: 10.3390/biomedicines11020301. Biomedicines. 2023. PMID: 36830836 Free PMC article.
-
Structural Insights into the Interactions of Digoxin and Na+/K+-ATPase and Other Targets for the Inhibition of Cancer Cell Proliferation.Molecules. 2021 Jun 16;26(12):3672. doi: 10.3390/molecules26123672. Molecules. 2021. PMID: 34208576 Free PMC article.
-
Evaluation of Anti-Inflammatory Activity of the New Cardiotonic Steroid γ-Benzylidene Digoxin 8 (BD-8) in Mice.Cells. 2024 Sep 18;13(18):1568. doi: 10.3390/cells13181568. Cells. 2024. PMID: 39329752 Free PMC article.
-
Depth of the Steroid Core Location Determines the Mode of Na,K-ATPase Inhibition by Cardiotonic Steroids.Int J Mol Sci. 2021 Dec 9;22(24):13268. doi: 10.3390/ijms222413268. Int J Mol Sci. 2021. PMID: 34948068 Free PMC article.
References
-
- de Wardener H.E., Mills I.H., Clapham W.F., Hayter C.J. Studies on the efferent mechanism of the sodium diuresis which follows the administration of intravenous saline in the dog. Clin Sci. 1961;21:249–258. - PubMed
-
- de Bold A.J., Borenstein H.B., Veress A.T., Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extracts in rats. Life Sci. 1981;28(1):89–94. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

