The cGAS-STING Pathway: Novel Perspectives in Liver Diseases
- PMID: 33995425
- PMCID: PMC8117096
- DOI: 10.3389/fimmu.2021.682736
The cGAS-STING Pathway: Novel Perspectives in Liver Diseases
Abstract
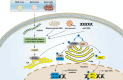
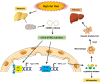
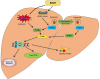
Liver diseases represent a major global health burden accounting for approximately 2 million deaths per year worldwide. The liver functions as a primary immune organ that is largely enriched with various innate immune cells, including macrophages, dendritic cells, neutrophils, NK cells, and NKT cells. Activation of these cells orchestrates the innate immune response and initiates liver inflammation in response to the danger signal from pathogens or injured cells and tissues. The cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway is a crucial signaling cascade of the innate immune system activated by cytosol DNA. Recognizing DNA as an immune-stimulatory molecule is an evolutionarily preserved mechanism in initiating rapid innate immune responses against microbial pathogens. The cGAS is a cytosolic DNA sensor eliciting robust immunity via the production of cyclic GMP-AMPs that bind and activate STING. Although the cGAS-STING pathway has been previously considered to have essential roles in innate immunity and host defense, recent advances have extended the role of the cGAS-STING pathway to liver diseases. Emerging evidence indicates that overactivation of cGAS-STING may contribute to the development of liver disorders, implying that the cGAS-STING pathway is a promising therapeutic target. Here, we review and discuss the role of the cGAS-STING DNA-sensing signaling pathway in a variety of liver diseases, including viral hepatitis, nonalcoholic fatty liver disease (NAFLD), alcoholic liver disease (ALD), primary hepatocellular cancer (HCC), and hepatic ischemia-reperfusion injury (IRI), with highlights on currently available therapeutic options.
Keywords: DNA sensor; Innate immunity; cyclic GMPAMP synthase; inflammation; liver diseases; stimulator of interferon genes.
Copyright © 2021 Xu, Tian, Xia and Ke.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The role of cGAS-STING signalling in liver diseases.JHEP Rep. 2021 Jun 24;3(5):100324. doi: 10.1016/j.jhepr.2021.100324. eCollection 2021 Oct. JHEP Rep. 2021. PMID: 34381984 Free PMC article. Review.
-
Conserved strategies for pathogen evasion of cGAS-STING immunity.Curr Opin Immunol. 2020 Oct;66:27-34. doi: 10.1016/j.coi.2020.04.002. Epub 2020 Apr 15. Curr Opin Immunol. 2020. PMID: 32339908 Free PMC article. Review.
-
The cGAS-STING pathway: The role of self-DNA sensing in inflammatory lung disease.FASEB J. 2020 Oct;34(10):13156-13170. doi: 10.1096/fj.202001607R. Epub 2020 Aug 28. FASEB J. 2020. PMID: 32860267 Free PMC article. Review.
-
The cGAS-STING Pathway in Bacterial Infection and Bacterial Immunity.Front Immunol. 2022 Jan 13;12:814709. doi: 10.3389/fimmu.2021.814709. eCollection 2021. Front Immunol. 2022. PMID: 35095914 Free PMC article. Review.
-
Salmonella Induces the cGAS-STING-Dependent Type I Interferon Response in Murine Macrophages by Triggering mtDNA Release.mBio. 2022 Jun 28;13(3):e0363221. doi: 10.1128/mbio.03632-21. Epub 2022 May 23. mBio. 2022. PMID: 35604097 Free PMC article.
Cited by
-
Emerging roles of liquid-liquid phase separation in liver innate immunity.Cell Commun Signal. 2024 Sep 3;22(1):430. doi: 10.1186/s12964-024-01787-4. Cell Commun Signal. 2024. PMID: 39227829 Free PMC article. Review.
-
Novel role of macrophage TXNIP-mediated CYLD-NRF2-OASL1 axis in stress-induced liver inflammation and cell death.JHEP Rep. 2022 Jul 8;4(9):100532. doi: 10.1016/j.jhepr.2022.100532. eCollection 2022 Sep. JHEP Rep. 2022. PMID: 36035360 Free PMC article.
-
Current understanding of the cGAS-STING signaling pathway: Structure, regulatory mechanisms, and related diseases.Zool Res. 2023 Jan 18;44(1):183-218. doi: 10.24272/j.issn.2095-8137.2022.464. Zool Res. 2023. PMID: 36579404 Free PMC article. Review.
-
Manganese Exacerbates ConA-Induced Liver Inflammation via the cGAS-STING Signaling Pathway.Inflammation. 2024 Feb;47(1):333-345. doi: 10.1007/s10753-023-01912-4. Epub 2023 Oct 8. Inflammation. 2024. PMID: 37805951
-
Scutellarin ameliorates ischemia/reperfusion injury‑induced cardiomyocyte apoptosis and cardiac dysfunction via inhibition of the cGAS‑STING pathway.Exp Ther Med. 2023 Feb 17;25(4):155. doi: 10.3892/etm.2023.11854. eCollection 2023 Apr. Exp Ther Med. 2023. PMID: 36911381 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

