Uveitis: Molecular Pathogenesis and Emerging Therapies
- PMID: 33995347
- PMCID: PMC8119754
- DOI: 10.3389/fimmu.2021.623725
Uveitis: Molecular Pathogenesis and Emerging Therapies
Abstract
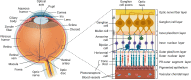
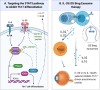
The profound impact that vision loss has on human activities and quality of life necessitates understanding the etiology of potentially blinding diseases and their clinical management. The unique anatomic features of the eye and its sequestration from peripheral immune system also provides a framework for studying other diseases in immune privileged sites and validating basic immunological principles. Thus, early studies of intraocular inflammatory diseases (uveitis) were at the forefront of research on organ transplantation. These studies laid the groundwork for foundational discoveries on how immune system distinguishes self from non-self and established current concepts of acquired immune tolerance and autoimmunity. Our charge in this review is to examine how advances in molecular cell biology and immunology over the past 3 decades have contributed to the understanding of mechanisms that underlie immunopathogenesis of uveitis. Particular emphasis is on how advances in biotechnology have been leveraged in developing biologics and cell-based immunotherapies for uveitis and other neuroinflammatory diseases.
Keywords: EAU; autoimmunity; cellular therapies; immunobiology; molecular therapies; uveitis.
Copyright © 2021 Egwuagu, Alhakeem and Mbanefo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Dracocephalum heterophyllum (DH) Exhibits Potent Anti-Proliferative Effects on Autoreactive CD4+ T Cells and Ameliorates the Development of Experimental Autoimmune Uveitis.Front Immunol. 2020 Oct 8;11:575669. doi: 10.3389/fimmu.2020.575669. eCollection 2020. Front Immunol. 2020. PMID: 33117376 Free PMC article.
-
Network pharmacology-based identification of the key mechanism of Qinghuo Rougan Formula acting on uveitis.Biomed Pharmacother. 2019 Dec;120:109381. doi: 10.1016/j.biopha.2019.109381. Epub 2019 Sep 19. Biomed Pharmacother. 2019. PMID: 31542616
-
Immune mechanisms in uveitis.Springer Semin Immunopathol. 1999;21(2):113-24. doi: 10.1007/BF00810244. Springer Semin Immunopathol. 1999. PMID: 10457585 Review. No abstract available.
-
[The status and prospects of immunological research in uveitis].Zhonghua Yan Ke Za Zhi. 2013 Mar;49(3):277-80. Zhonghua Yan Ke Za Zhi. 2013. PMID: 23866710 Review. Chinese.
-
Kallistatin Attenuates Experimental Autoimmune Uveitis by Inhibiting Activation of T Cells.Front Immunol. 2020 May 21;11:975. doi: 10.3389/fimmu.2020.00975. eCollection 2020. Front Immunol. 2020. PMID: 32508841 Free PMC article.
Cited by
-
Morroniside ameliorates lipopolysaccharide-induced inflammatory damage in iris pigment epithelial cells through inhibition of TLR4/JAK2/STAT3 pathway.Int J Ophthalmol. 2023 Dec 18;16(12):1928-1934. doi: 10.18240/ijo.2023.12.03. eCollection 2023. Int J Ophthalmol. 2023. PMID: 38111933 Free PMC article.
-
Identification of uveitis-associated functions based on the feature selection analysis of gene ontology and Kyoto Encyclopedia of Genes and Genomes pathway enrichment scores.Front Mol Neurosci. 2022 Sep 8;15:1007352. doi: 10.3389/fnmol.2022.1007352. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36157069 Free PMC article.
-
Causal role of immune cells in uveitis: Mendelian randomization study.Front Immunol. 2024 Jul 9;15:1402074. doi: 10.3389/fimmu.2024.1402074. eCollection 2024. Front Immunol. 2024. PMID: 39044820 Free PMC article.
-
Perspectives of Therapeutic Drug Monitoring of Biological Agents in Non-Infectious Uveitis Treatment: A Review.Pharmaceutics. 2023 Feb 25;15(3):766. doi: 10.3390/pharmaceutics15030766. Pharmaceutics. 2023. PMID: 36986627 Free PMC article. Review.
-
Adhesion Molecule Targeted Therapy for Non-Infectious Uveitis.Int J Mol Sci. 2022 Jan 3;23(1):503. doi: 10.3390/ijms23010503. Int J Mol Sci. 2022. PMID: 35008929 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

